Chapter 1 Nature of Physical Chemistry Physical Chemistry





- Slides: 13

Chapter 1. Nature of Physical Chemistry


Physical Chemistry Principle Subject Areas • Thermodynamics 熱力學 • Quantum Chemistry 量子力學 • Chemical Kinetics 化學動力學 Intermediate Areas • Electrochemistry 電化學 • Statistic thermodynamics 統計熱力學 Application Area • Spectroscopy 光譜學 • Solid State chemistry 固態化學 • Surface chemistry 表面化學


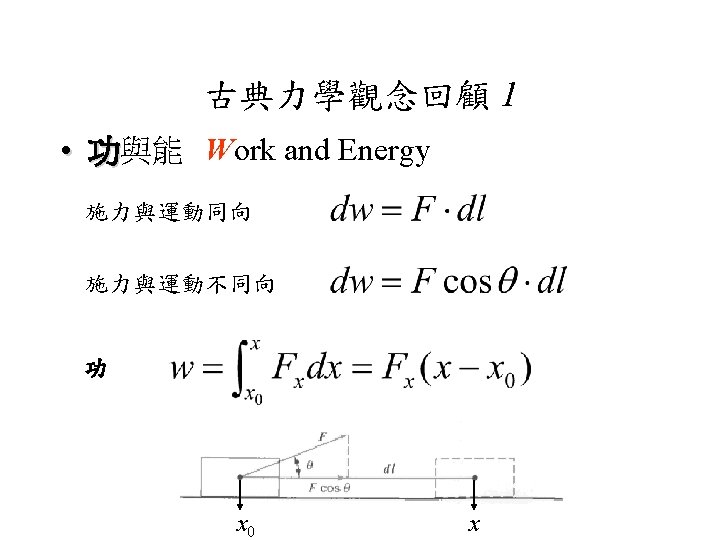
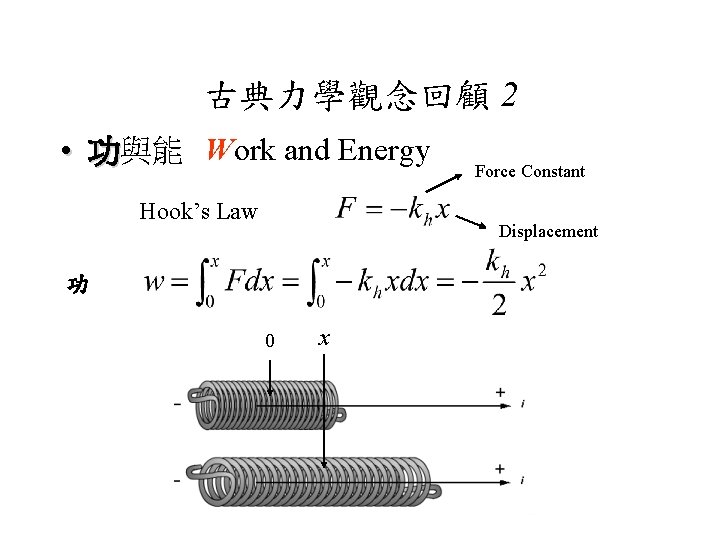
古典力學觀念回顧 2 • 功與能 Work and Energy Hook’s Law Force Constant Displacement 功 0 x

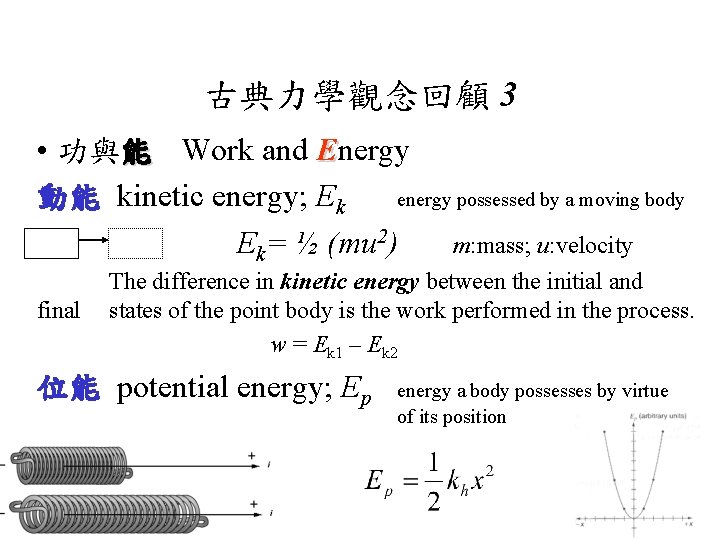
古典力學觀念回顧 3 • 功與能 Work and Energy 動能 kinetic energy; Ek energy possessed by a moving body Ek= ½ (mu 2) m: mass; u: velocity final The difference in kinetic energy between the initial and states of the point body is the work performed in the process. w = Ek 1 – Ek 2 位能 potential energy; Ep energy a body possesses by virtue of its position

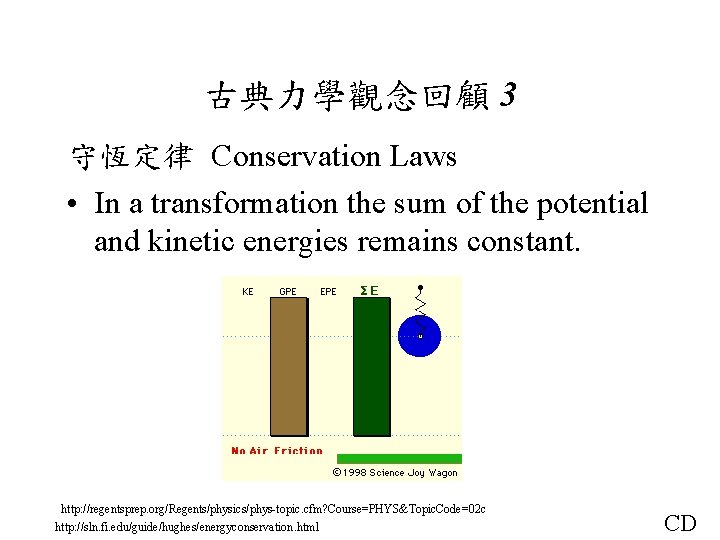
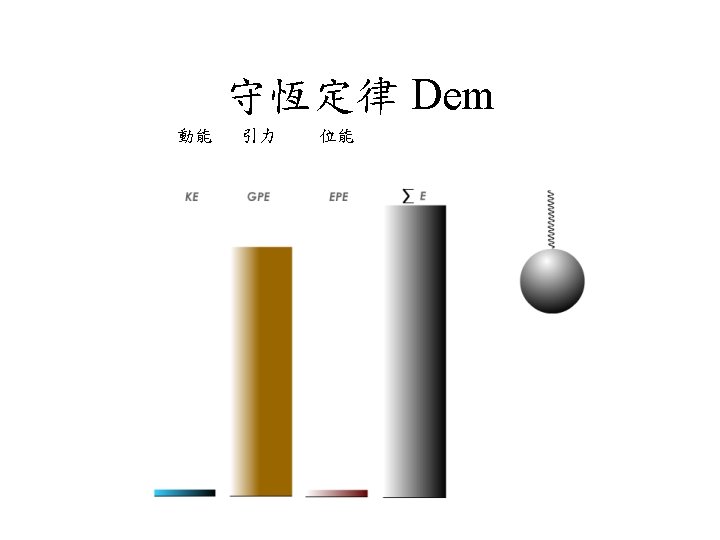
古典力學觀念回顧 3 守恆定律 Conservation Laws • In a transformation the sum of the potential and kinetic energies remains constant. http: //regentsprep. org/Regents/physics/phys-topic. cfm? Course=PHYS&Topic. Code=02 c http: //sln. fi. edu/guide/hughes/energyconservation. html CD



示強與示量 intensive & extensive • 示強性質 Intensive Propertie The property does not change with the quantity of matter present Example: density, molar heat • 示量性質 Extensive Properties The property does change with the quantity of matter present Example: mass, volume The ratio of two extensive properties is an intensive properties

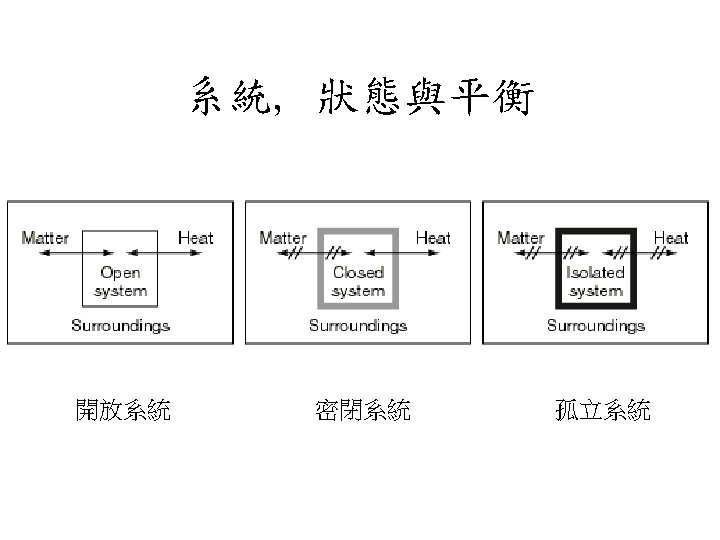
狀態與平衡 State & Equilibrium • 狀態 State For a given amount of material it is usually possibel to use an equation to describe the states in terms of intensive variables • 平衡 Equilibrium The variables that specify the state of the system do not change with time.

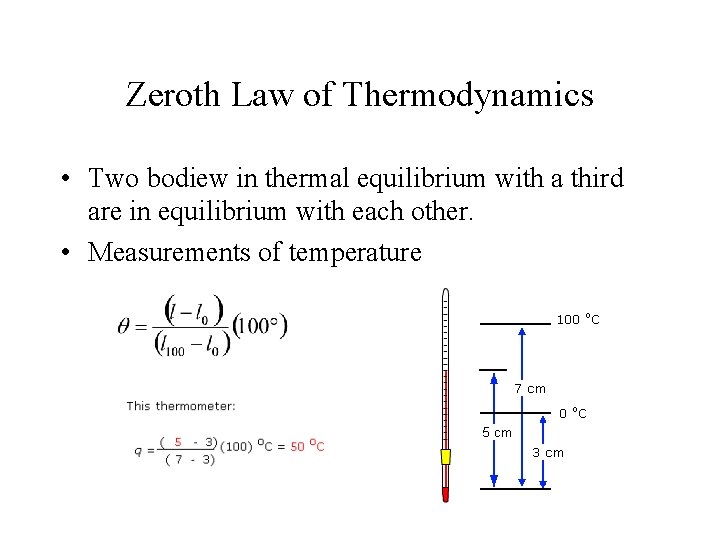
Zeroth Law of Thermodynamics • Two bodiew in thermal equilibrium with a third are in equilibrium with each other. • Measurements of temperature

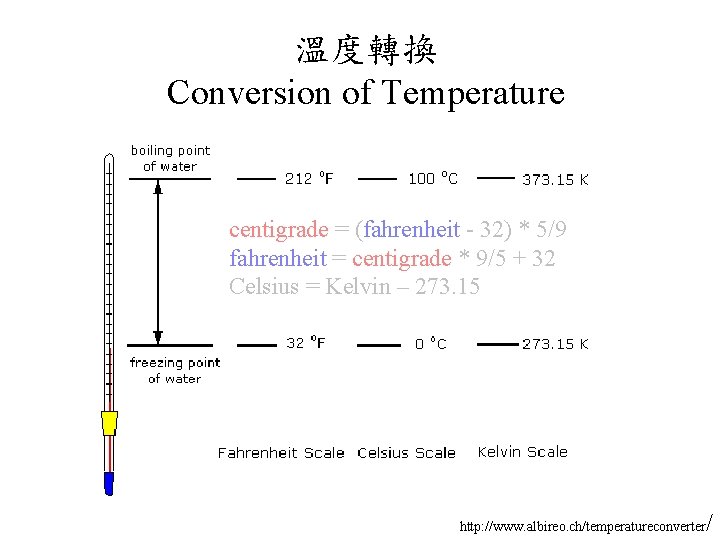
溫度轉換 Conversion of Temperature centigrade = (fahrenheit - 32) * 5/9 fahrenheit = centigrade * 9/5 + 32 Celsius = Kelvin – 273. 15 http: //www. albireo. ch/temperatureconverter /