Chapter 1 Matter anything that has mass and





- Slides: 8

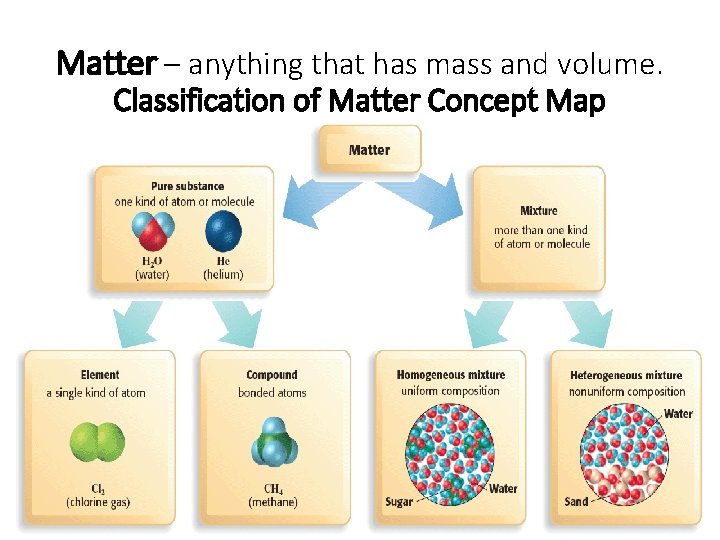
Chapter 1 Matter – anything that has mass and volume. Classification of Matter Concept Map • Classifying Matter

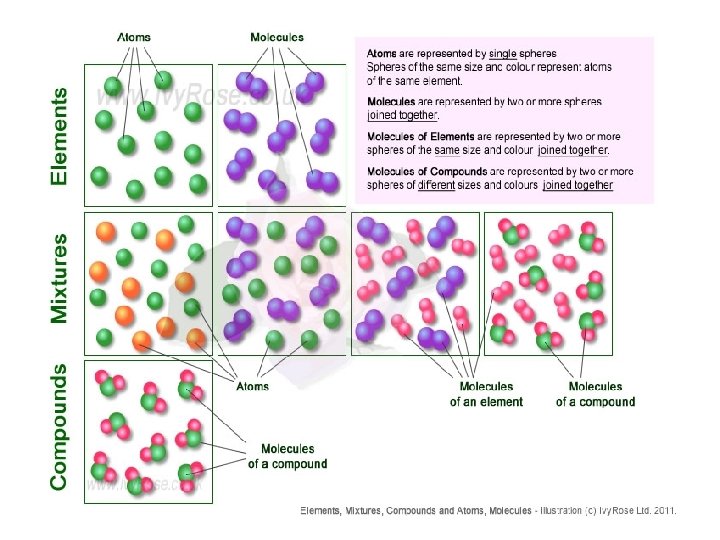
Matter can be classified into: Pure substance is a kind of matter that cannot be separated into other kinds of matter by any physical process (Only one substance) An example of a pure substance is pure silver. Mixture – a combination of 2 or more substances that are NOT chemically bonded. An example of a mixture is a mixed vegetable salad. Another example of a mixture is pure gold + copper

Pure substances can be classified into Element and Compound: Element – only has one kind of atom e. g. Na (sodium) Compound – more than one kind of atom e. g. Na. Cl (sodium chloride)

• Element – a substance that cannot be broken down into simpler substance by chemical means (found in periodic table == > more than 100 kinds) • An example of an element is pure gold • Atom – the smallest unit of an element that still has all of the properties of that element (building block of matter)

• Compound – two or more kinds of atoms of different elements that are chemically bound together. • An example of a compound is H 2 O (water). • Molecule – the smallest unit of a compound that has all of the properties of that compound.

• Mixtures can be classified into: • Homogeneous (or called solutions) • Mixtures which are the same throughout with identical properties everywhere in the mixture. • Not easily separated. • An example of this would be salt water (Can’t separate salt from water easily) • Heterogeneous • Mixtures which have different properties when sampled from different areas. • Examples of this would be sand mixed with water or peanuts mixed with raisins (Can separate peanuts from raisins easily)

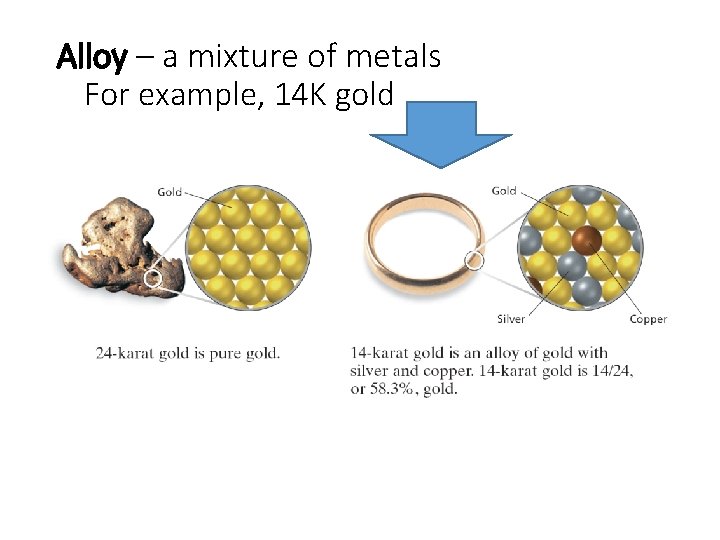
Alloy – a mixture of metals For example, 14 K gold
