Chapter 1 Matter and Measurement Why study chemistry









- Slides: 22

Chapter 1 – Matter and Measurement Why study chemistry?

Matter and Measurement • Chemistry is the study of matter and how it interacts with other types of matter.

Matter and Measurement • Matter consists of elements or compounds. • Element • Compound

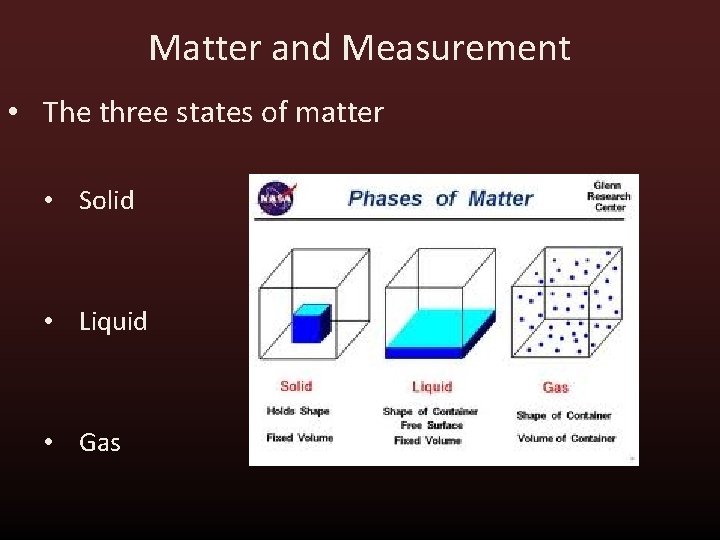
Matter and Measurement • The three states of matter • Solid • Liquid • Gas

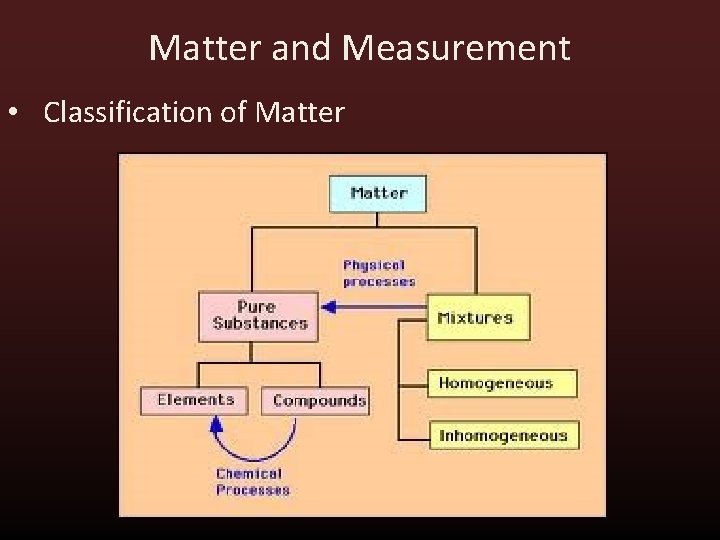
Matter and Measurement • Classification of Matter

Matter and Measurement • Properties of Matter • Physical Properties – characteristics that can be observed without changing the identity of the substance.

Matter and Measurement • Properties of Matter • Chemical Properties – describes how a substance can change by reacting with another substance.

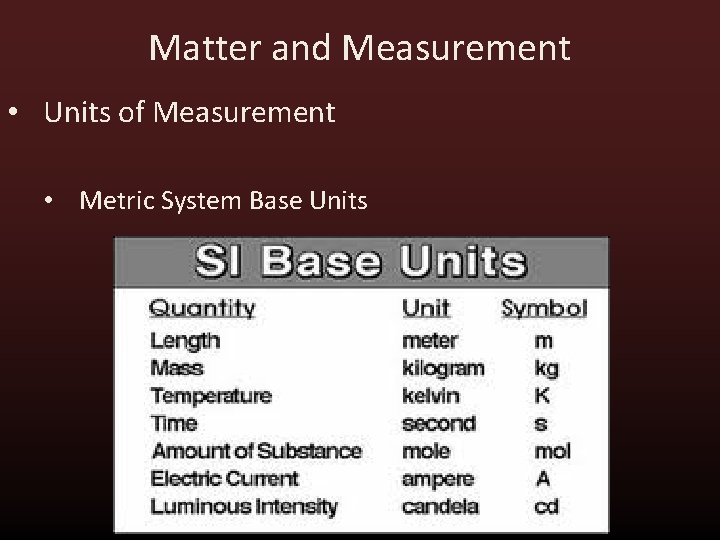
Matter and Measurement • Units of Measurement • Metric System - Adopted in 1960. Derived from the SI system (Systeme International d’Unites).

Matter and Measurement • Units of Measurement • Metric System Base Units

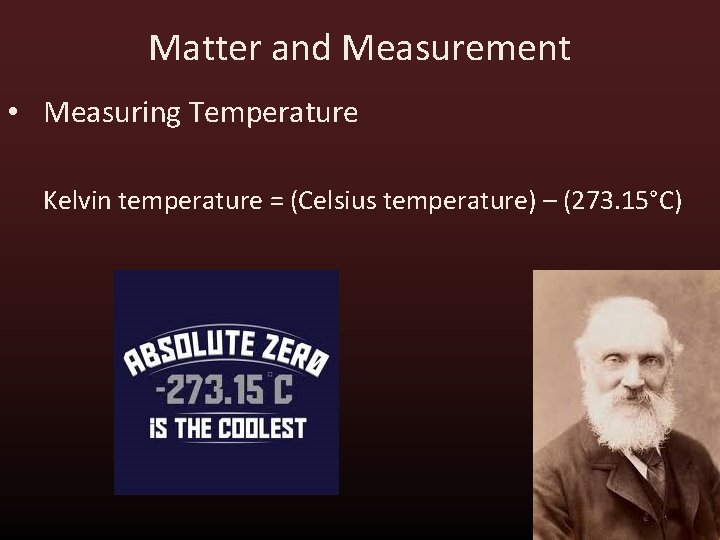
Matter and Measurement • Measuring Temperature • Many units for measuring temperature exist. • Lord Kelvin discovered theoretical coldest temperature of matter. 0 Kelvin (Absolute Zero) 0 Kelvin = -273. 15°C

Matter and Measurement • Measuring Temperature Kelvin temperature = (Celsius temperature) – (273. 15°C)

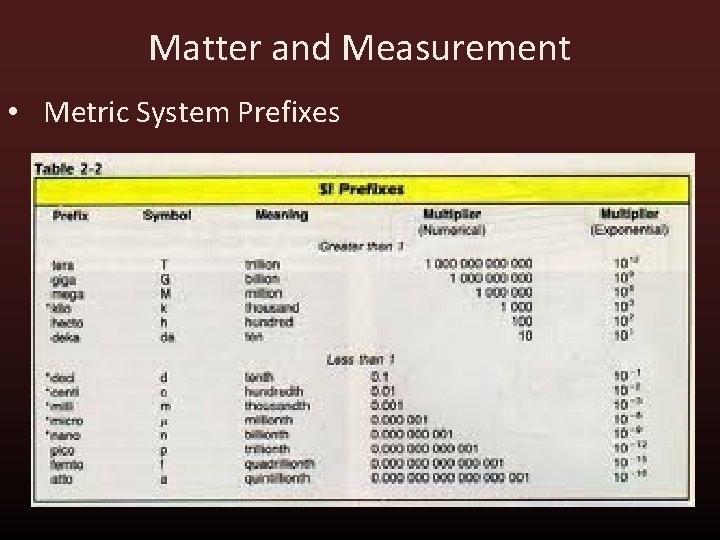
Matter and Measurement • Metric System Prefixes

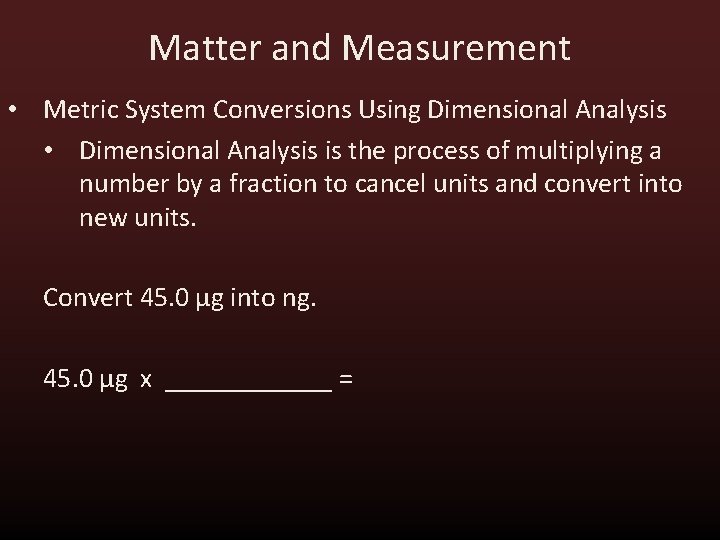
Matter and Measurement • Metric System Conversions Using Dimensional Analysis • Dimensional Analysis is the process of multiplying a number by a fraction to cancel units and convert into new units. Convert 45. 0 μg into ng. 45. 0 μg x ______ =

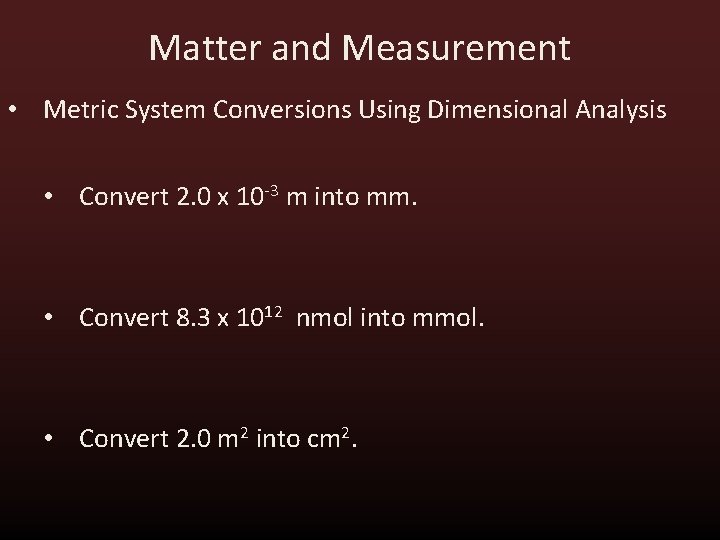
Matter and Measurement • Metric System Conversions Using Dimensional Analysis • Convert 2. 0 x 10 -3 m into mm. • Convert 8. 3 x 1012 nmol into mmol. • Convert 2. 0 m 2 into cm 2.

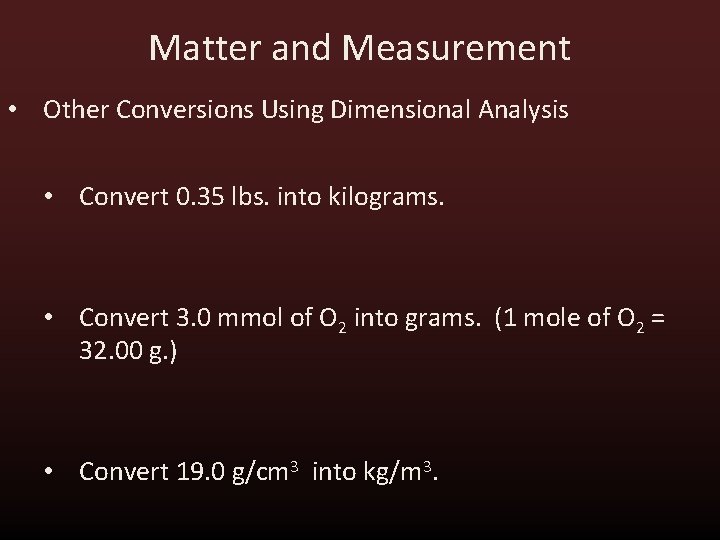
Matter and Measurement • Other Conversions Using Dimensional Analysis • Convert 0. 35 lbs. into kilograms. • Convert 3. 0 mmol of O 2 into grams. (1 mole of O 2 = 32. 00 g. ) • Convert 19. 0 g/cm 3 into kg/m 3.

Matter and Measurement • Significant Figures – “You can only be as accurate as your least accurate instrument. ” • A significant figure is a measured value. • All nonzero numbers are significant. • Zeros are sometimes significant. • Captive zeros are significant. (203 m) 3 s. f. • Leading zeros are not significant. • Trailing zeros are significant if the number contains a decimal point. (450 s) 2 s. f. (1021. 00 μmol) 6 s. f (0. 002 g) 1 s. f.

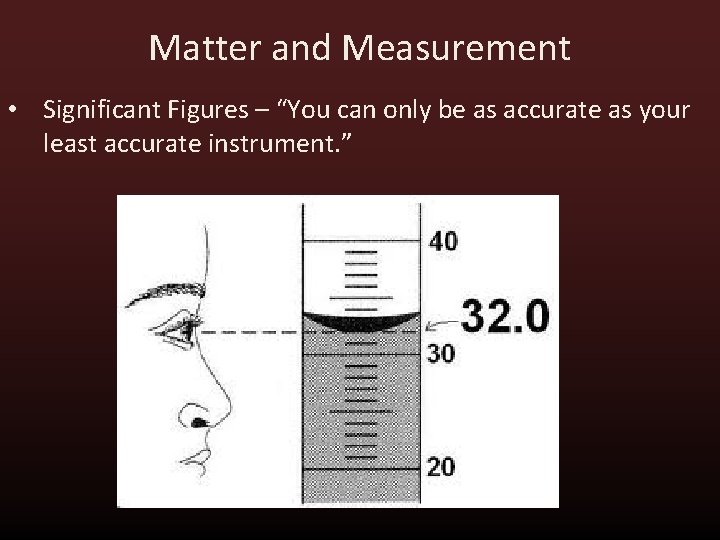
Matter and Measurement • Significant Figures – “You can only be as accurate as your least accurate instrument. ”

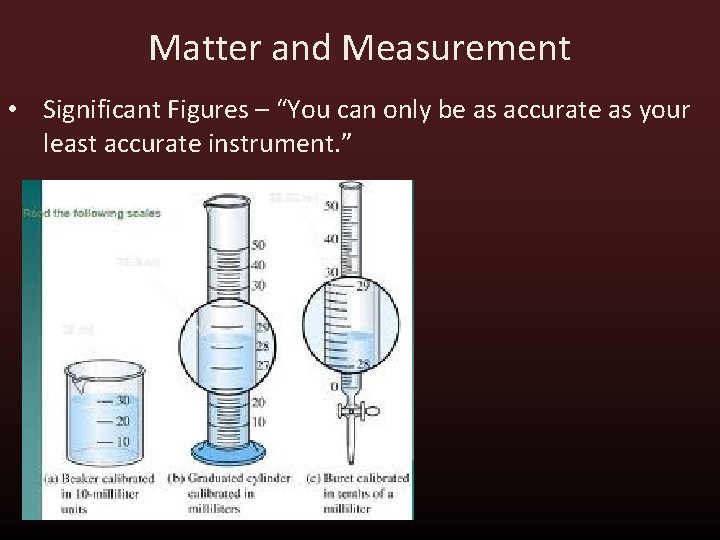
Matter and Measurement • Significant Figures – “You can only be as accurate as your least accurate instrument. ”

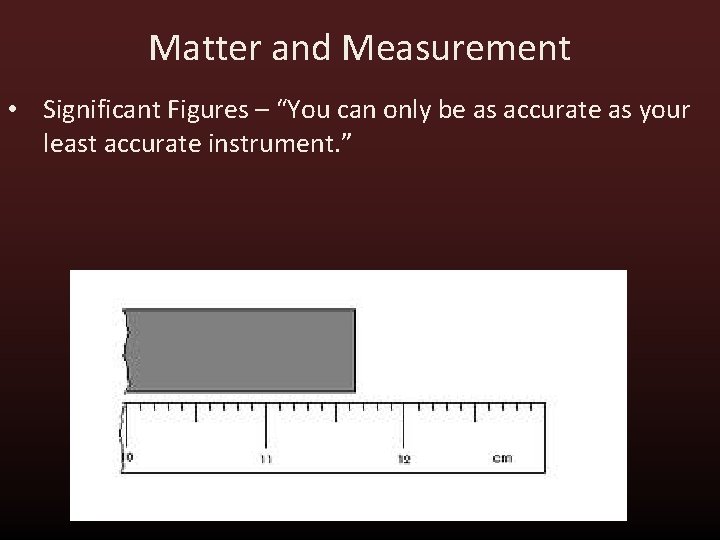
Matter and Measurement • Significant Figures – “You can only be as accurate as your least accurate instrument. ”

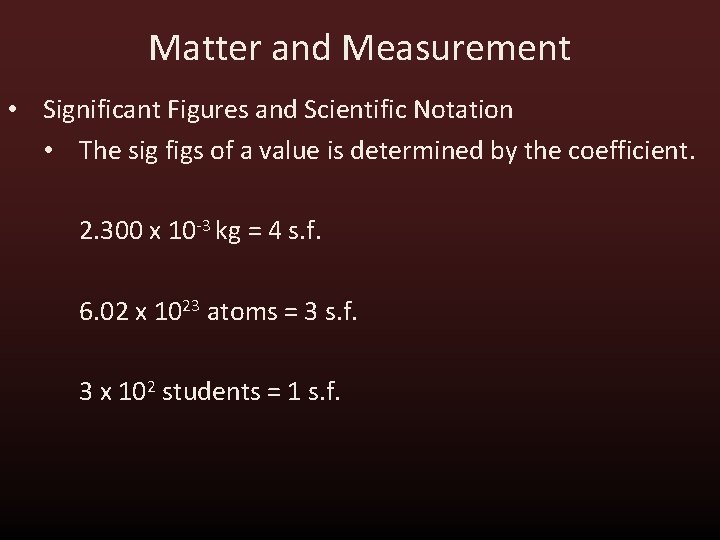
Matter and Measurement • Significant Figures and Scientific Notation • The sig figs of a value is determined by the coefficient. 2. 300 x 10 -3 kg = 4 s. f. 6. 02 x 1023 atoms = 3 s. f. 3 x 102 students = 1 s. f.

Matter and Measurement • Significant Figures and Calculations • Multiplication and Division – Answer is rounded to the same number of sig figs as the number with the least sig figs in the problem. 45 m x 2. 00 m = 9. 0 x 101 m 2 or 90. m 2 3. 500 g / 25. 0 g = 0. 140

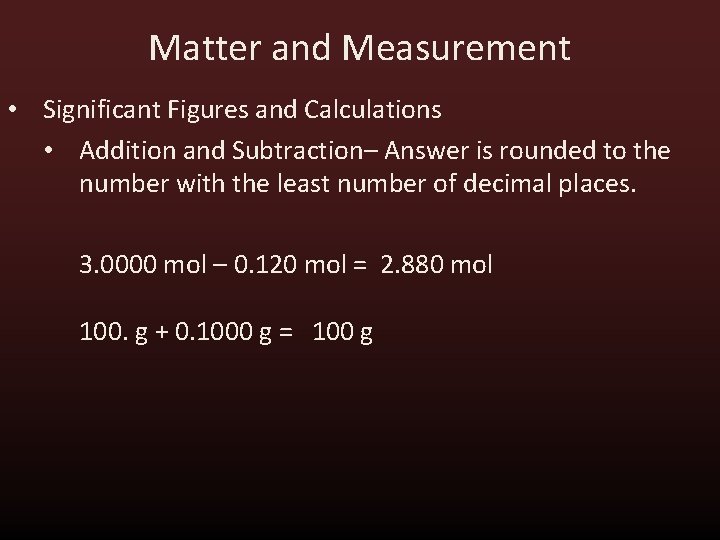
Matter and Measurement • Significant Figures and Calculations • Addition and Subtraction– Answer is rounded to the number with the least number of decimal places. 3. 0000 mol – 0. 120 mol = 2. 880 mol 100. g + 0. 1000 g = 100 g