Chapter 1 Matter and Change Section 1 1


























- Slides: 39

Chapter 1: Matter and Change

Section 1. 1: Chemistry is a Physical Science • Physical science focuses on nonliving things • Chemistry is the study of the composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany these processes

• Instruments are used to observe make and measurements • Electron microscope beams electrons at materials – Hit a material, scatter and produce a pattern that shows the material’s microstructure • X-rays form X-ray diffraction patterns to reveal the arrangement of atoms, molecules, or other particles

Branches of Chemistry • Organic chemistry- study most carboncontaining compounds • Inorganic chemistry- study non-organic substances, many of which have organic fragments bonded to metals • Physical chemistry- study properties and changes of matter and their relation to energy

• Analytical chemistry- the identification of the components and composition of materials • Biochemistry- the study of substances and processes occurring in living things • Theoretical chemistry- the use of mathematics and computers to understand the principles behind observed chemical behavior and to design and predict he properties of new compounds

• Chemical- any substance that has a definite composition – Sucrose – Carbon dioxide, water • Knowing the properties allows chemists to find uses for them

• Basic research- how and why a reaction occurs and what the properties are • Chance discoveries • Applied research is carried out to solve a problem – Not driven by curiosity but to solve a problem

• Technological development- produce and use of products that improve our quality of life – Computers, converters, biodegradable materials • Application lags far behind the discoveries

• Basic research, applied research, and technological development often overlap

Section 1. 2 Matter and Its Properties • Mass is a measure of the amount of matter • Matter is anything that has mass and takes up space

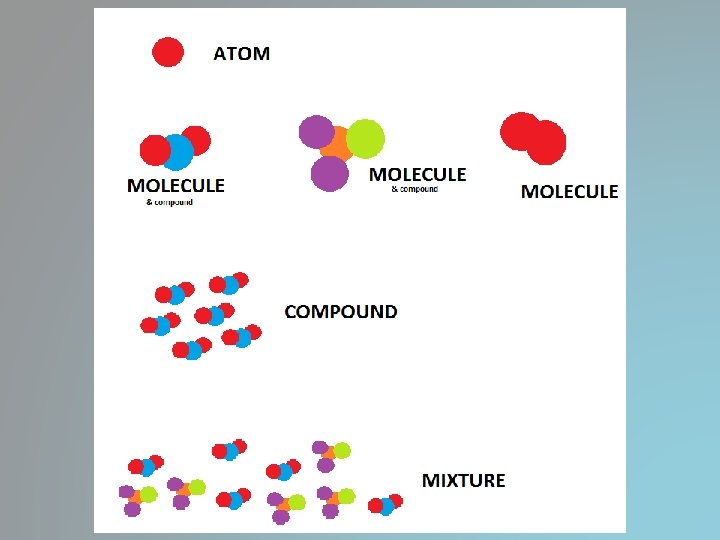
Basic Building Blocks of Matter • Atom is the smallest unit of an element that maintains the chemical identity of that element • Elements is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom

• Compound is a substance that can be broken down into simple stable substances – Made of 2 or more elements that are chemically bonded – H 2 O • Molecule is the smallest unit of an element or compound that retains all of the properties of that element or compound


Properties and Changes in Matter • Every element or compound has characteristic properties • Properties reveal the identity of an unknown substance • Comparison of several properties can be used to establish the identity

• Extensive properties depend on the amount of matter that is present – Volume, mass, and amount of energy in a substance • Intensive properties do not depend on the amount of matter present – Melting point, boiling point, density, ability to conduct electricity and transfer hear

• Physical property- characteristic that can be observed or measured without changing the identity of the substance – Describes the substance itself, rather than describing how it can change into other substances – Melting point and boiling point

• Physical change- a change in a substance that does not involve a change in the identity of the substance – Grinding, cutting, melting, boiling • Change of state- physical change of a substance from one state to another

• Solid- state has definite volume and definite shape – Keeps its size and shape, particles tightly packed • Liquid- has volume but indefinite shape – Particles are close together but move past one another • Gas- neither volume nor definite shape – Particles move rapidly and great distances apart

• Plasma- high-temperature physical state of matter in which atoms lose most of their electrons • What causes changes in matter?

Chemical properties and changes • Chemical property relates to a substance’s ability to undergo changes that transform it into different substances • A change in which one or more substances are converted into different substances is a chemical change

In a chemical reaction, we have: • Reactants- substances that react in a chemical change • Products are formed by the chemical change carbon + oxygen carbon dioxide • Arrows mean yield • Whenever there is chemical changes, energy is involved – Light, heat , gas

Classifying Matter • Mixtures-a blend of two or more kinds of matter, each of which retains its own identity and properties – Parts can be physically separated – Pretty much everything is a mixture

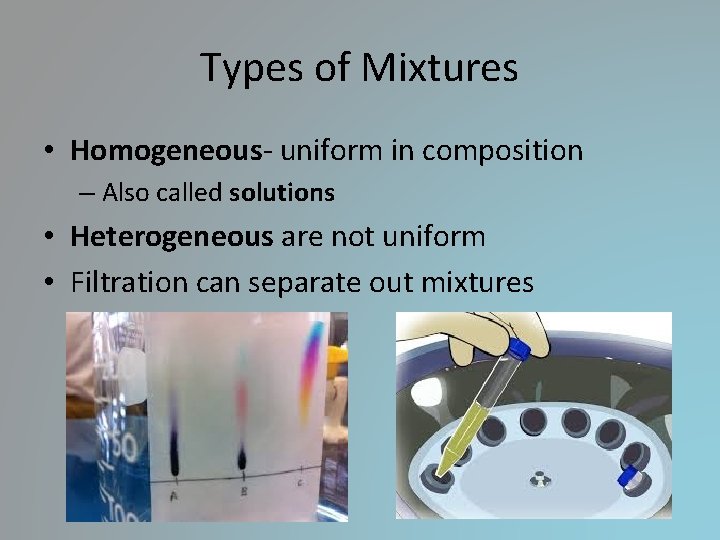
Types of Mixtures • Homogeneous- uniform in composition – Also called solutions • Heterogeneous are not uniform • Filtration can separate out mixtures

Pure Substances • Pure substances have a fixed composition and differs from a mixture in the following ways – Every sample has the exact same characteristic and properties – Every sample has exactly the same composition – Either compounds or elements • Compounds can be broken down elctrolysis

Elements

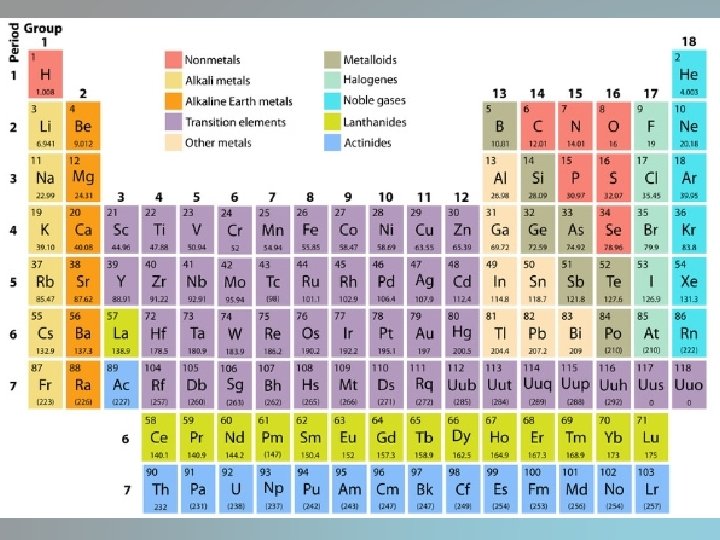
The periodic table • Vertical columns are called groups or families • Organized based on similar chemical properties • Two major groups: metals and nonmetals – Metalloids have properties intermediate between • Periods are the horizontal rows of elements – Physical and chemical properties change across a period – Elements close to each other have similar properties

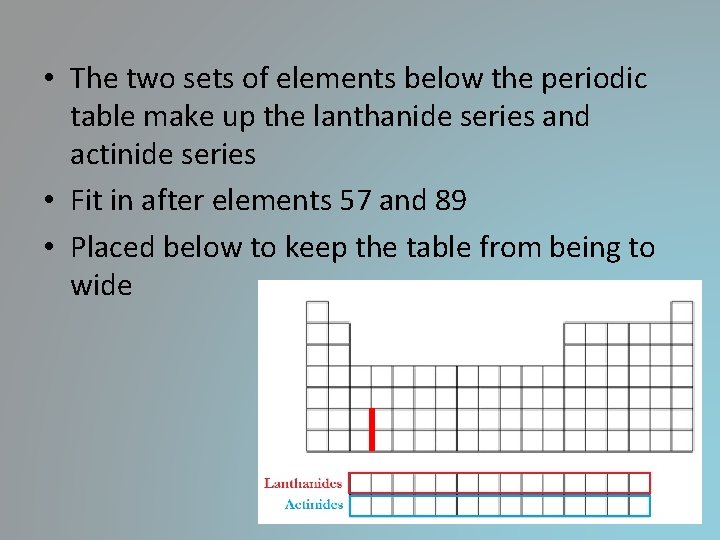
• The two sets of elements below the periodic table make up the lanthanide series and actinide series • Fit in after elements 57 and 89 • Placed below to keep the table from being to wide

Types of Elements • Metals – Shiny, metallic luster – Conduct electricity and transfer energy – Most are metals at room temperature – Malleable- can be hammered or rolled into thin sheets – Ductile- drawn into a fine wire – Tensile strength- the ability to resist breaking

• Nonmetals – Most are gases at room temperature – Some are liquids – Others are solids – Poor conductor of heat and electricity

• Metalloids – Found between the metals and nonmetals – Have some characteristics of metals and nonmetals – Solids at room temperature – Less malleable than metals – Brittle like nonmetals – Semiconductors of electricity

• Noble gases – Group 18 – Uncreative – Gases at room temperature


Alkali metals • • • Very reactive Do not occur naturally in nature Softer than most metals Can explode if exposed to water https: //www. youtube. com/watch? v=uixx. Jt. JPV Xk

Alkaline Earth Metals • Very reactive • Not found free in nature

Transition Elements • 38 elements • Valence electrons are present in more than one shell • Often exhibit several oxidation states

Other Metals • Valence electrons stay in outer shell • Have oxidation numbers of +3, ± 4, and -3 • High density and opaque

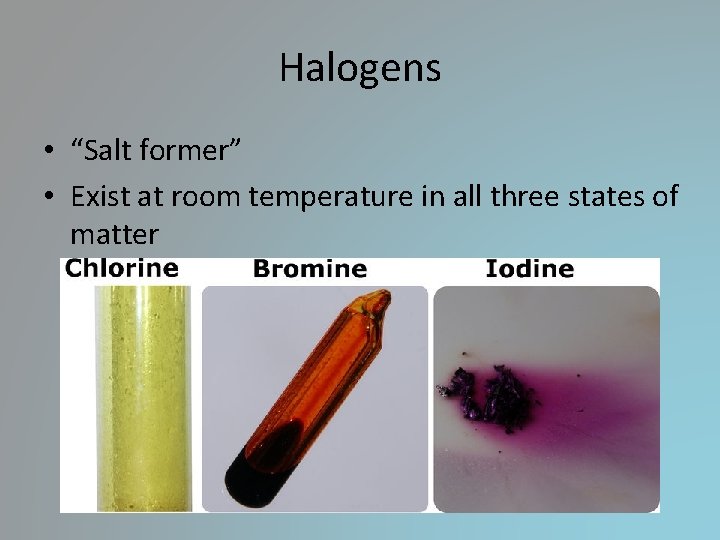
Halogens • “Salt former” • Exist at room temperature in all three states of matter

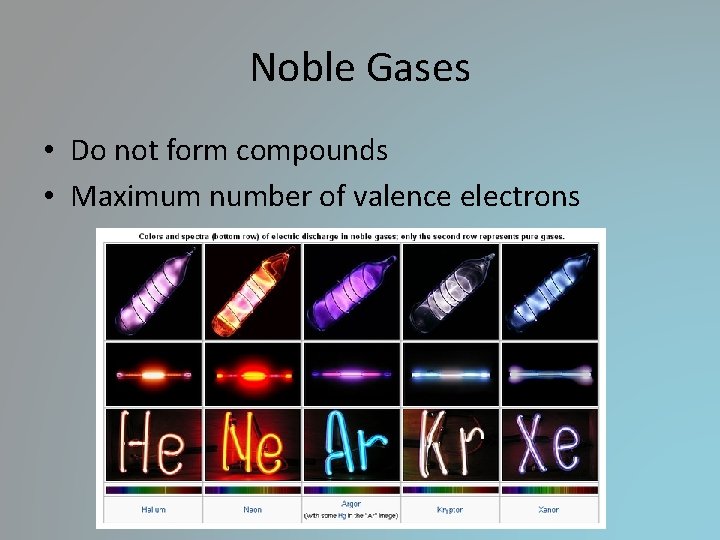
Noble Gases • Do not form compounds • Maximum number of valence electrons

Rare Earth Elements • Lanthanide • Actinide • One element of the lanthanide series and most of the elements in the actinide series are called trans-uranium, which means synthetic or man-made