Chapter 1 lecture 2 ME 203 1 Properties

- Slides: 19

Chapter 1 - lecture 2 ME 203 1

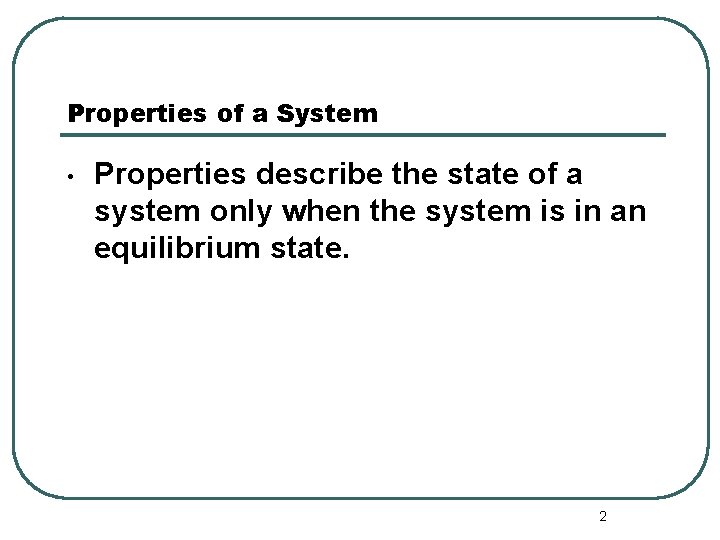
Properties of a System • Properties describe the state of a system only when the system is in an equilibrium state. 2

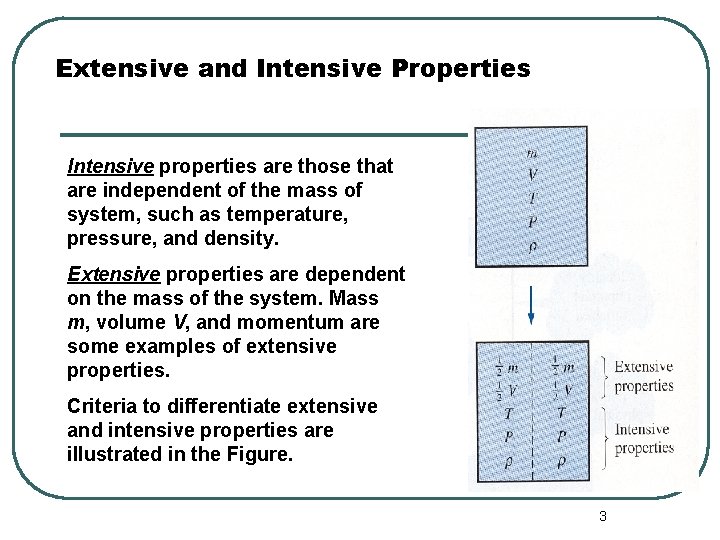
Extensive and Intensive Properties Intensive properties are those that are independent of the mass of system, such as temperature, pressure, and density. Extensive properties are dependent on the mass of the system. Mass m, volume V, and momentum are some examples of extensive properties. Criteria to differentiate extensive and intensive properties are illustrated in the Figure. 3

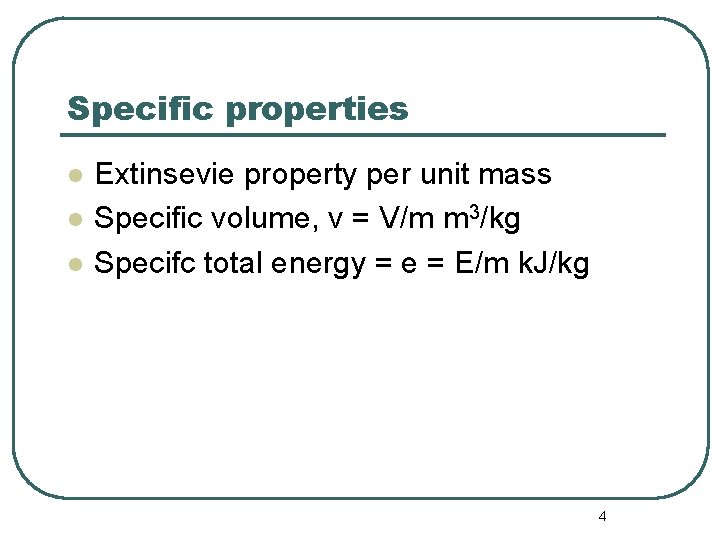
Specific properties l l l Extinsevie property per unit mass Specific volume, v = V/m m 3/kg Specifc total energy = e = E/m k. J/kg 4

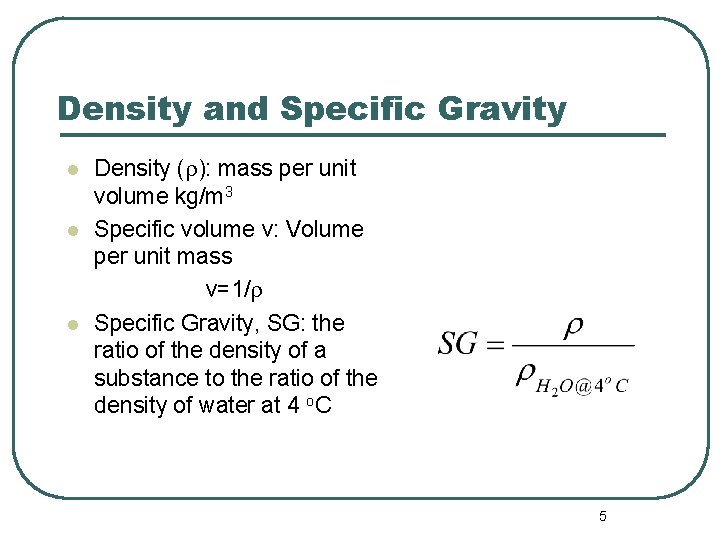
Density and Specific Gravity l l l Density (r): mass per unit volume kg/m 3 Specific volume v: Volume per unit mass v=1/r Specific Gravity, SG: the ratio of the density of a substance to the ratio of the density of water at 4 o. C 5

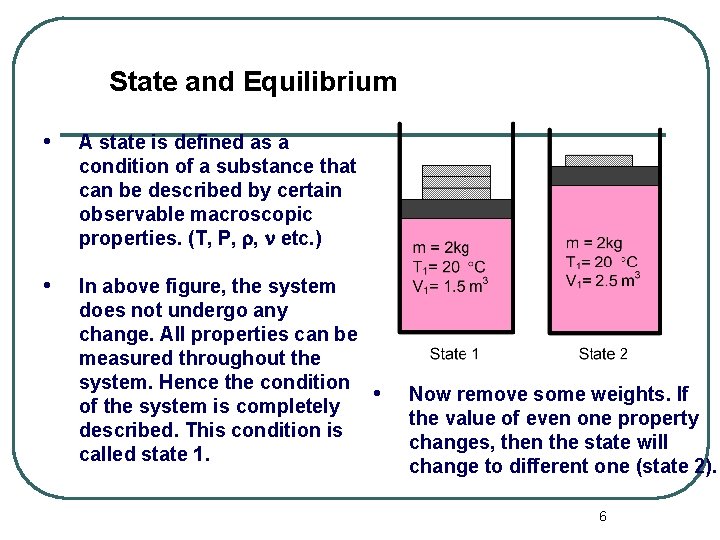
State and Equilibrium • A state is defined as a condition of a substance that can be described by certain observable macroscopic properties. (T, P, , etc. ) • In above figure, the system does not undergo any change. All properties can be measured throughout the system. Hence the condition of the system is completely described. This condition is called state 1. • Now remove some weights. If the value of even one property changes, then the state will change to different one (state 2). 6

State and Equilibrium • Thermodynamics deals with equilibrium states. • The word equilibrium implies a state of balance. • Equilibrium state means that there are no unbalanced potentials (or driving forces) within the system. • A system is said to be in thermodynamic equilibrium if it maintains thermal, mechanical, phase, and chemical equilibrium. 7

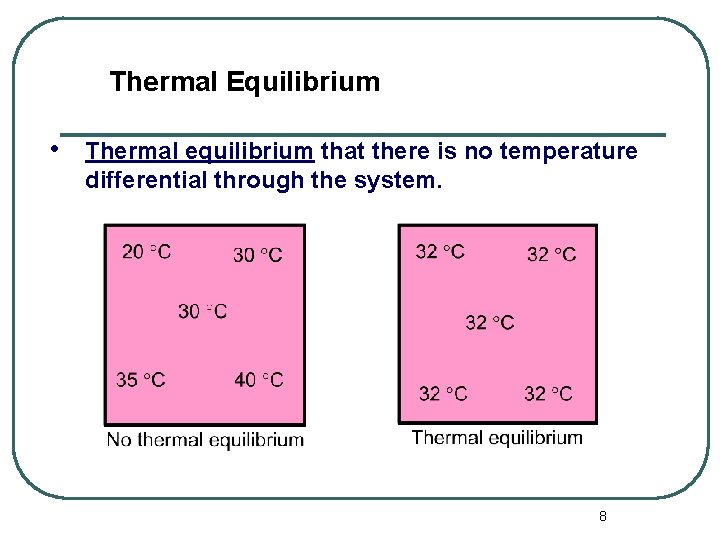
Thermal Equilibrium • Thermal equilibrium that there is no temperature differential through the system. 8

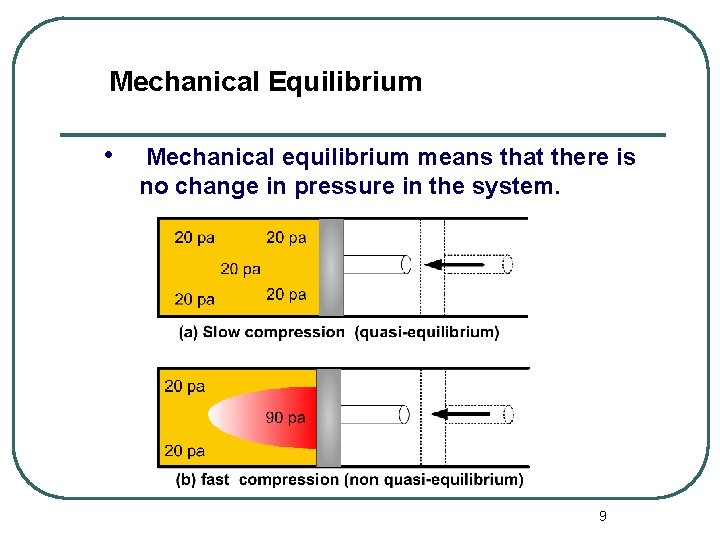
Mechanical Equilibrium • Mechanical equilibrium means that there is no change in pressure in the system. 9

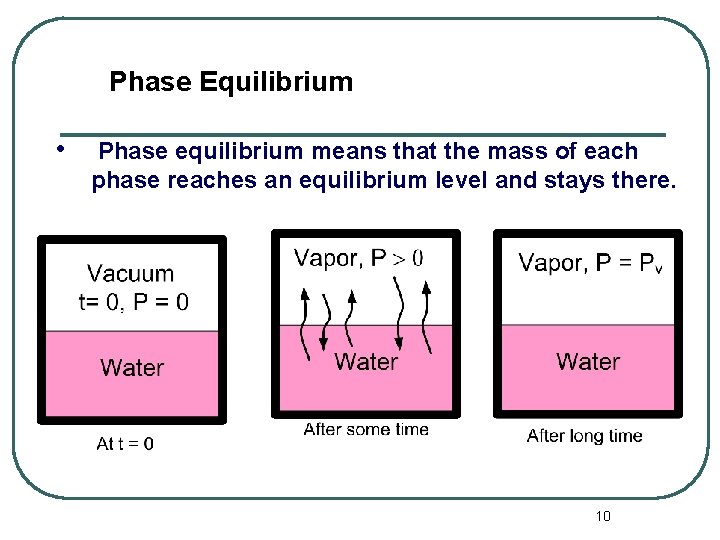
Phase Equilibrium • Phase equilibrium means that the mass of each phase reaches an equilibrium level and stays there. 10

Chemical Equilibrium • Chemical equilibrium means that the chemical composition of the system does not change with time 11

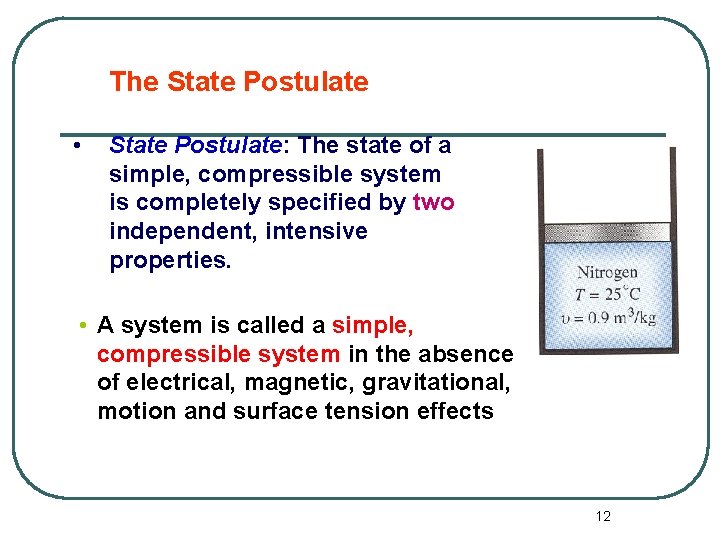
The State Postulate • State Postulate: The state of a simple, compressible system is completely specified by two independent, intensive properties. • A system is called a simple, compressible system in the absence of electrical, magnetic, gravitational, motion and surface tension effects 12

The State Postulate (continued) • Temperature and specific volume, for example, are always independent properties, and together they can fix the state of a simple compressible system. • Temperature and pressure, however, are independent properties for single -phase systems, but are dependent properties for multiphase systems. 13

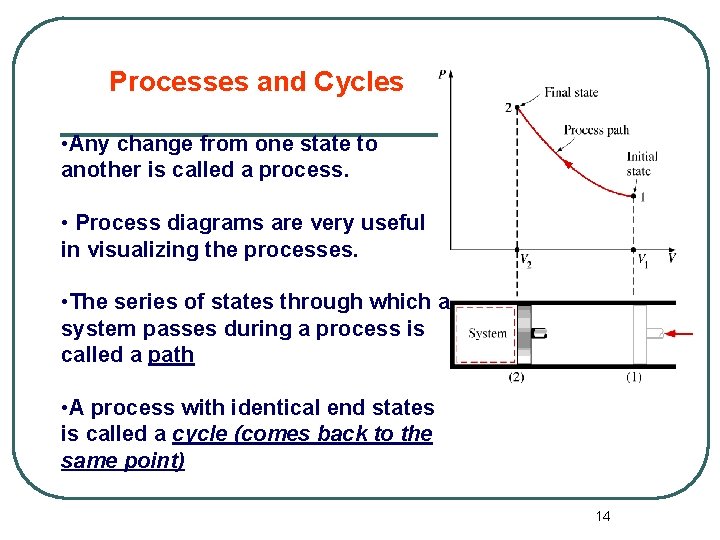
Processes and Cycles • Any change from one state to another is called a process. • Process diagrams are very useful in visualizing the processes. • The series of states through which a system passes during a process is called a path • A process with identical end states is called a cycle (comes back to the same point) 14

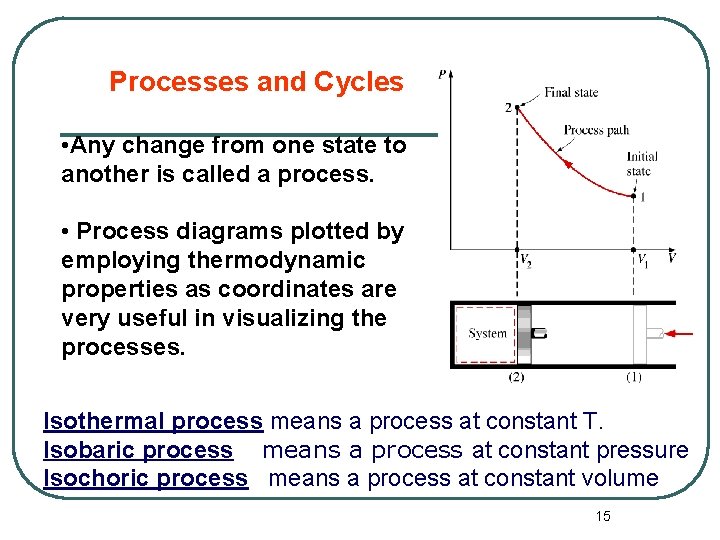
Processes and Cycles • Any change from one state to another is called a process. • Process diagrams plotted by employing thermodynamic properties as coordinates are very useful in visualizing the processes. Isothermal process means a process at constant T. Isobaric process means a process at constant pressure Isochoric process means a process at constant volume 15

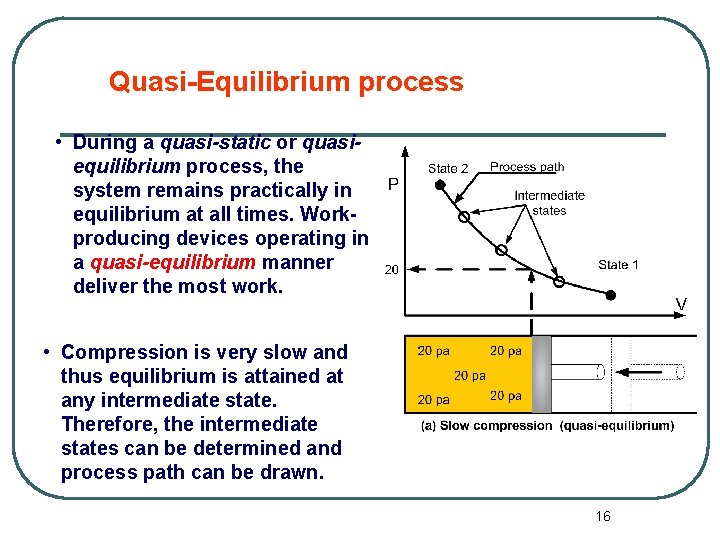
Quasi-Equilibrium process • During a quasi-static or quasiequilibrium process, the system remains practically in equilibrium at all times. Workproducing devices operating in a quasi-equilibrium manner deliver the most work. • Compression is very slow and thus equilibrium is attained at any intermediate state. Therefore, the intermediate states can be determined and process path can be drawn. 16

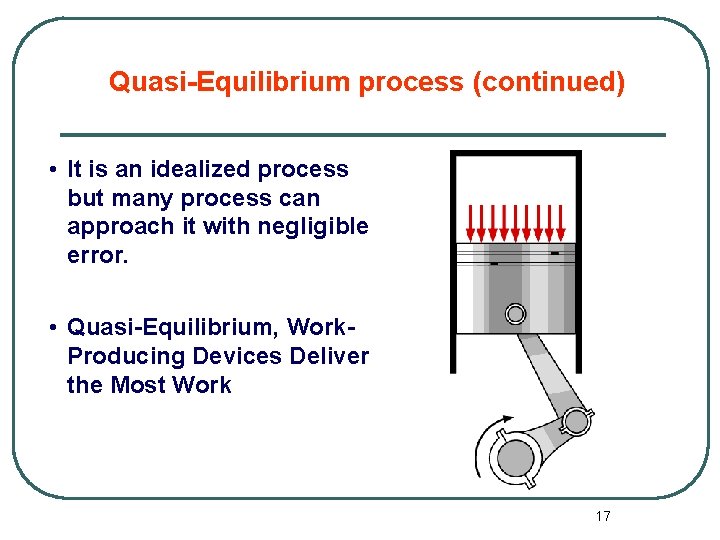
Quasi-Equilibrium process (continued) • It is an idealized process but many process can approach it with negligible error. • Quasi-Equilibrium, Work. Producing Devices Deliver the Most Work 17

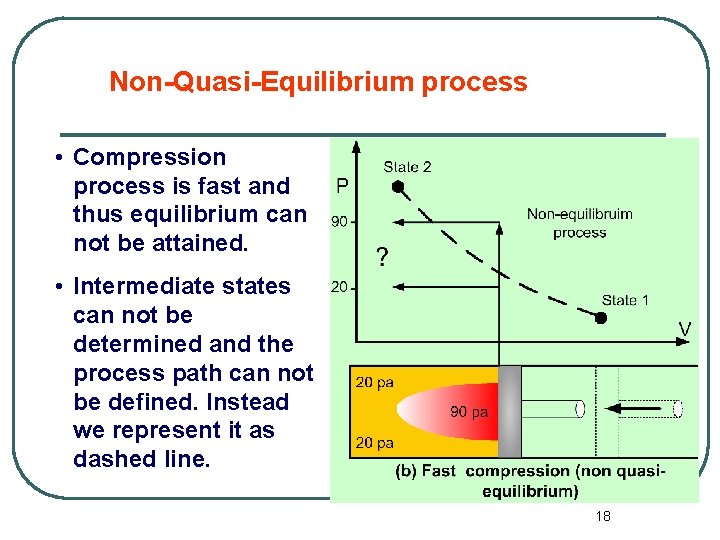
Non-Quasi-Equilibrium process • Compression process is fast and thus equilibrium can not be attained. • Intermediate states can not be determined and the process path can not be defined. Instead we represent it as dashed line. 18

The Steady Flow Process l l l Steady: no change with time (in = out), d/dt =0 ( time derivative = 0) Unsteady or Transient : there is a change with time d/dt does not equal zero Uniform: no change with locations over a specific region d/dx = 0 19