CHAPTER 1 Kinetic Particle Theory 2013 Marshall Cavendish
















- Slides: 60

CHAPTER 1 Kinetic Particle Theory © 2013 Marshall Cavendish International (Singapore) Private Limited

Chapter 1 Kinetic Particle Theory 1. 1 States of Matter 1. 2 Kinetic Particle Theory 1. 3 Changes in State of Matter and the Kinetic Particle Theory 1. 4 Diffusion 2

1. 1 States of Matter Learning Outcome At the end of this section, you should be able to: • describe the solid, liquid and gaseous state of matter. 3

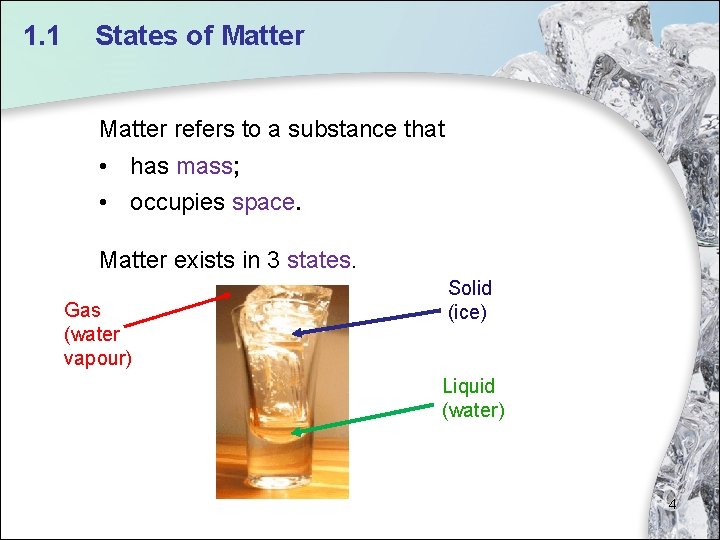
1. 1 States of Matter refers to a substance that • has mass; • occupies space. Matter exists in 3 states. Gas (water vapour) Solid (ice) Liquid (water) 4

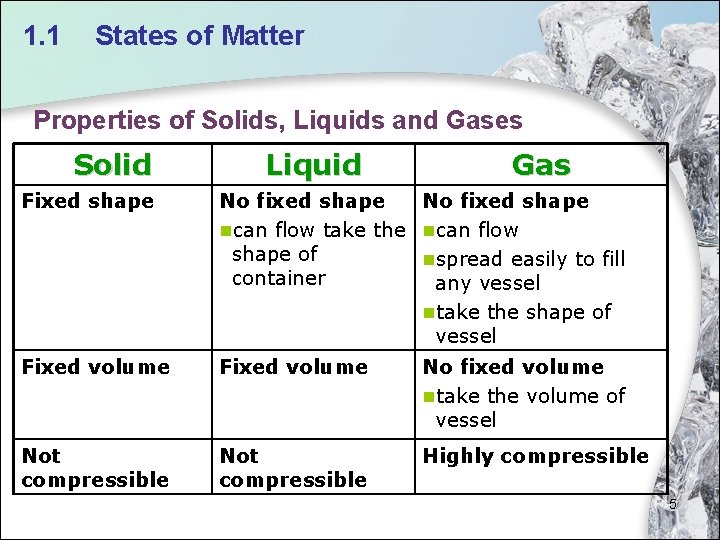
1. 1 States of Matter Properties of Solids, Liquids and Gases Solid Liquid Gas Fixed shape No fixed shape ncan flow take the ncan flow shape of nspread easily to fill container any vessel ntake the shape of vessel Fixed volume No fixed volume ntake the volume of vessel Not compressible Highly compressible 5

Chapter 1 Kinetic Particle Theory 1. 1 States of Matter 1. 2 Kinetic Particle Theory 1. 3 Changes in State of Matter and the Kinetic Particle Theory 1. 4 Diffusion 6

1. 2 Kinetic Particle Theory Learning Outcomes At the end of this section, you should be able to: • state the kinetic particle theory; • describe the states of matter using the kinetic particle theory. 7

1. 2 Kinetic Particle Theory Are Particles in Constant Motion? The ‘dancing’ dust that you see in a beam of light is actually the result of air particles moving and bumping into dust! Air particles are too small to be seen by our eyes, therefore we can only see the dust moving. The explanations for the ‘dancing’ dust are based on the kinetic particle theory. 8

1. 2 Kinetic Particle Theory What is Kinetic Particle Theory? The kinetic particle theory states that: Atoms, molecules, ions • All matter is made up of tiny particles. • Particles are in constant and random motion (and thus possess kinetic energy), colliding with one another. • Constant = non–stop, continuous • Random = unpredictable • Speed of particles depend on the amount of kinetic energy 9

1. 2 Kinetic Particle Theory What is Kinetic Particle Theory? The fundamental difference between solids, liquids and gases is the degree of movement of their particles. Refer to the Video “States of Matter” http: //www. youtube. com/watch? v=s. Kvo. Vzuk. Ho 10

1. 2 Kinetic Particle Theory Consider the following properties in the three states of matter: Kinetic energy of particles Forces of attraction between particles Movement of particles Arrangement of particles 11

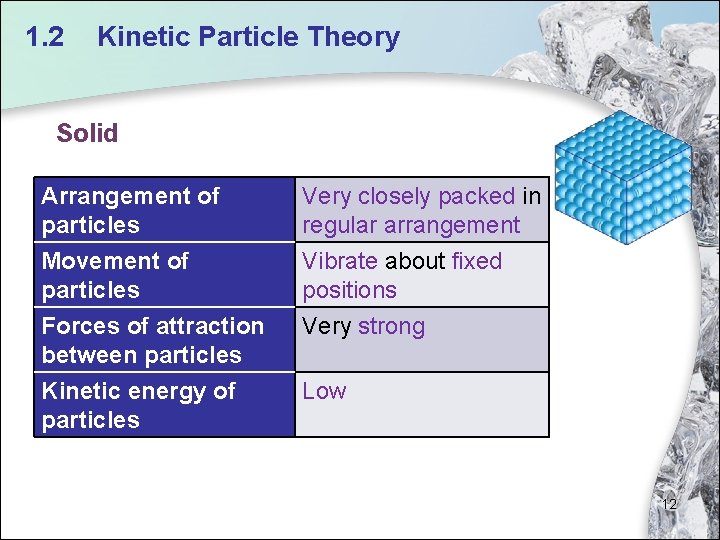
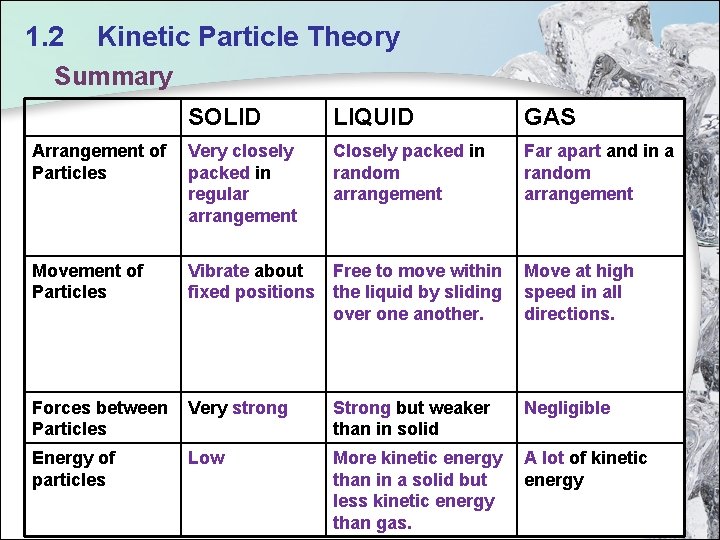
1. 2 Kinetic Particle Theory Solid Arrangement of particles Very closely packed in regular arrangement Movement of particles Vibrate about fixed positions Forces of attraction between particles Very strong Kinetic energy of particles Low 12

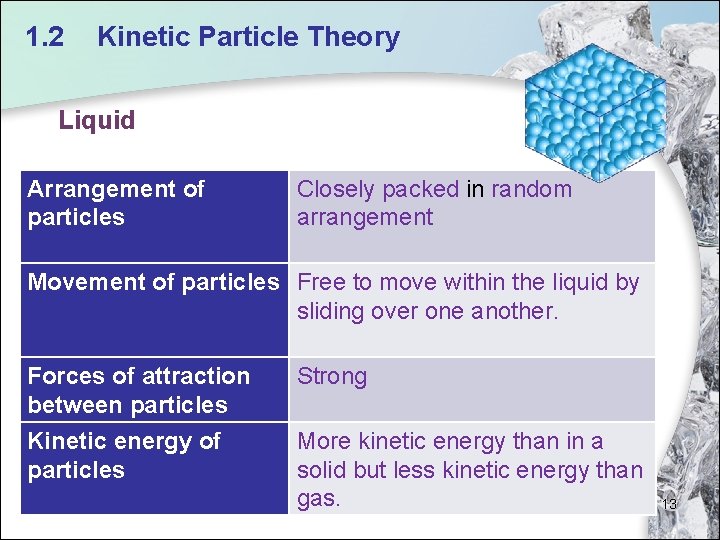
1. 2 Kinetic Particle Theory Liquid Arrangement of particles Closely packed in random arrangement Movement of particles Free to move within the liquid by sliding over one another. Forces of attraction between particles Strong Kinetic energy of particles More kinetic energy than in a solid but less kinetic energy than gas. 13

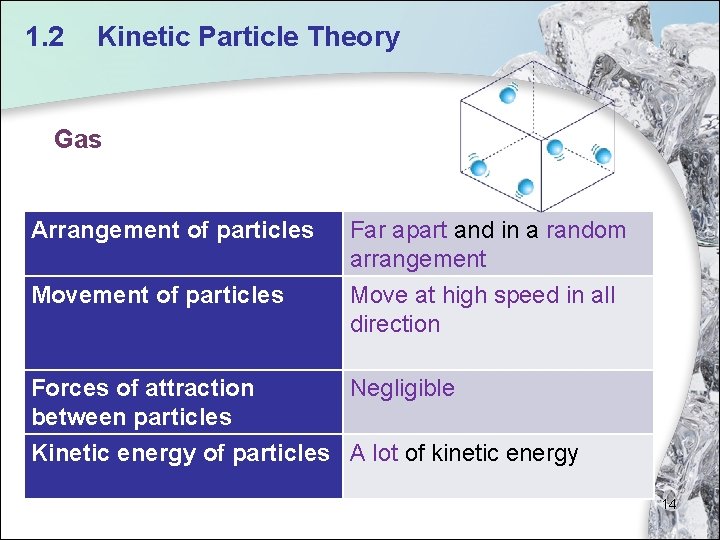
1. 2 Kinetic Particle Theory Gas Arrangement of particles Movement of particles Far apart and in a random arrangement Move at high speed in all direction Forces of attraction Negligible between particles Kinetic energy of particles A lot of kinetic energy 14

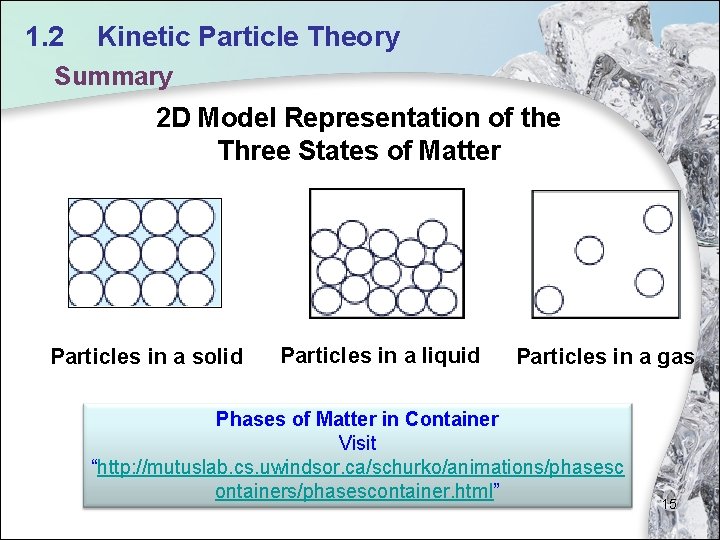
1. 2 Kinetic Particle Theory Summary 2 D Model Representation of the Three States of Matter Particles in a solid Particles in a liquid Particles in a gas Phases of Matter in Container Visit “http: //mutuslab. cs. uwindsor. ca/schurko/animations/phasesc ontainers/phasescontainer. html” 15

1. 2 Kinetic Particle Theory Summary SOLID LIQUID GAS Arrangement of Particles Very closely packed in regular arrangement Closely packed in random arrangement Far apart and in a random arrangement Movement of Particles Vibrate about Free to move within fixed positions the liquid by sliding over one another. Forces between Particles Very strong Strong but weaker than in solid Negligible Energy of particles Low More kinetic energy than in a solid but less kinetic energy than gas. A lot of kinetic energy Move at high speed in all directions. 16

Chapter 1 Kinetic Particle Theory 1. 1 States of Matter 1. 2 Kinetic Particle Theory 1. 3 Changes in State of Matter and the Kinetic Particle Theory 1. 4 Diffusion 17

1. 3 Changes in State of Matter and the Kinetic Particle Theory Learning Outcome At the end of this section, you should be able to: • explain the inter-conversion of matter in terms of the kinetic particle theory and energy changes. • sketch of graphs to show the transition of physical states of substances e. g. boiling, melting, freezing, condensation processes 18

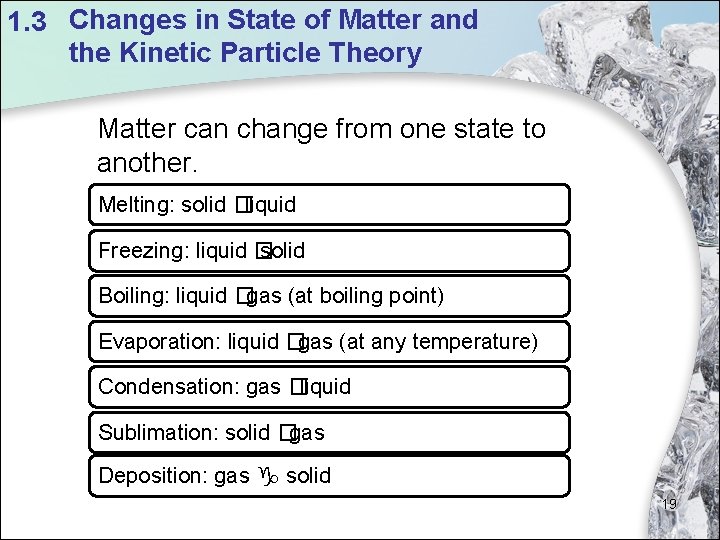
1. 3 Changes in State of Matter and the Kinetic Particle Theory Matter can change from one state to another. Melting: solid �liquid Freezing: liquid � solid Boiling: liquid �gas (at boiling point) Evaporation: liquid �gas (at any temperature) Condensation: gas �liquid Sublimation: solid �gas Deposition: gas solid 19

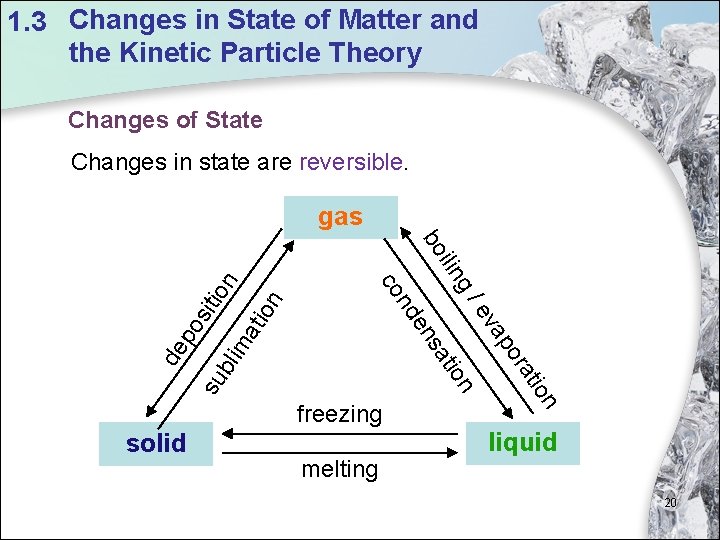
1. 3 Changes in State of Matter and the Kinetic Particle Theory Changes of State Changes in state are reversible. tio n ma bli po s de su melting on ati or ap ev n tio sa freezing solid g/ ilin bo n de n co itio n gas liquid 20

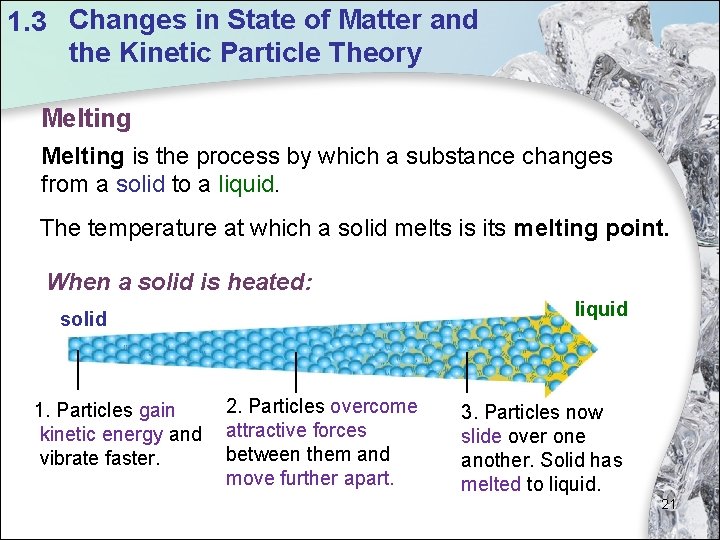
1. 3 Changes in State of Matter and the Kinetic Particle Theory Melting is the process by which a substance changes from a solid to a liquid. The temperature at which a solid melts is its melting point. When a solid is heated: liquid solid 1. Particles gain kinetic energy and vibrate faster. 2. Particles overcome attractive forces between them and move further apart. 3. Particles now slide over one another. Solid has melted to liquid. 21

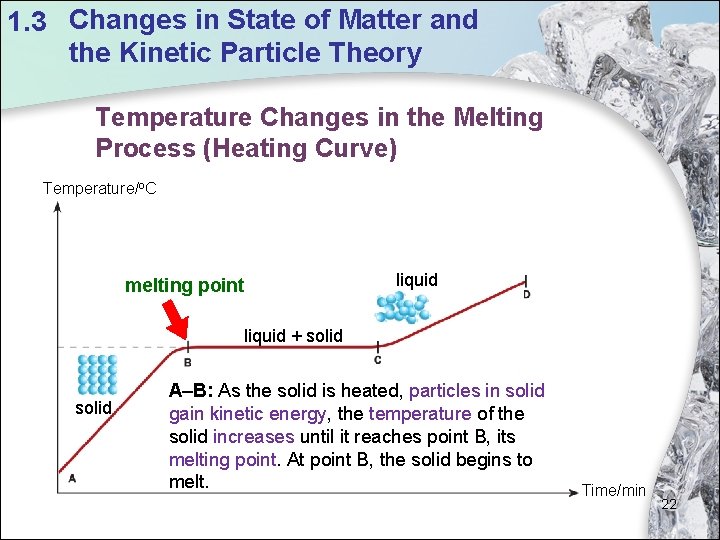
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Melting Process (Heating Curve) Temperature/o. C melting point liquid + solid A–B: As the solid is heated, particles in solid gain kinetic energy, the temperature of the solid increases until it reaches point B, its melting point. At point B, the solid begins to melt. Time/min 22

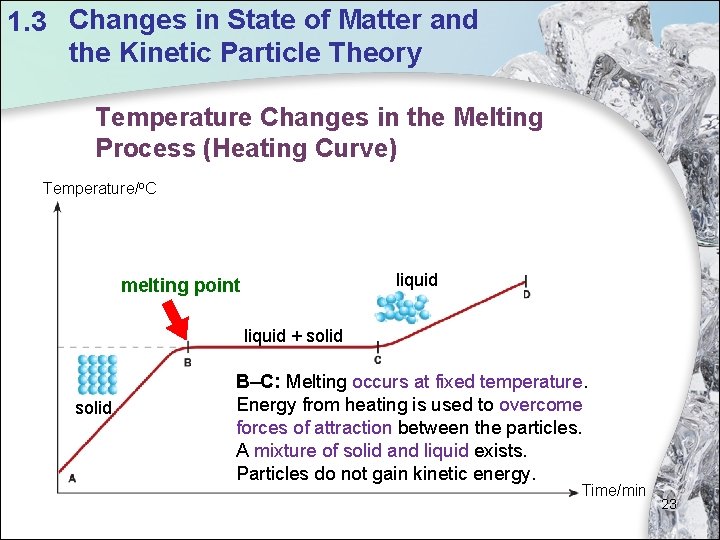
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Melting Process (Heating Curve) Temperature/o. C liquid melting point liquid + solid B–C: Melting occurs at fixed temperature. Energy from heating is used to overcome forces of attraction between the particles. A mixture of solid and liquid exists. Particles do not gain kinetic energy. Time/min 23

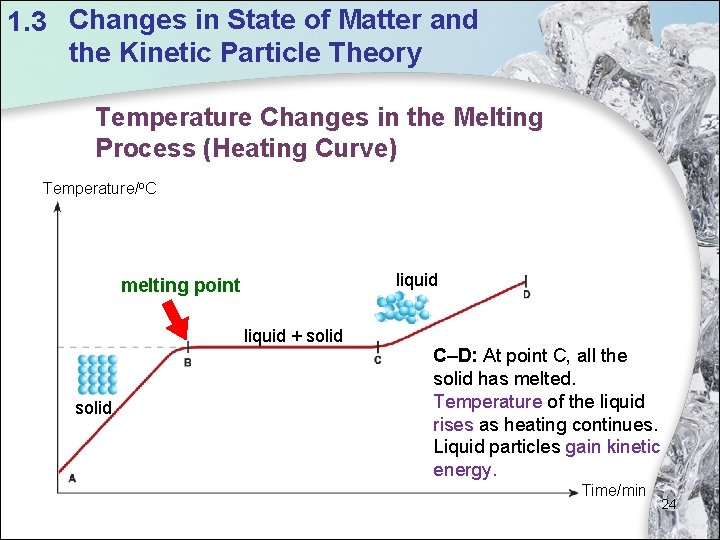
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Melting Process (Heating Curve) Temperature/o. C liquid melting point liquid + solid C–D: At point C, all the solid has melted. Temperature of the liquid rises as heating continues. Liquid particles gain kinetic energy. Time/min 24

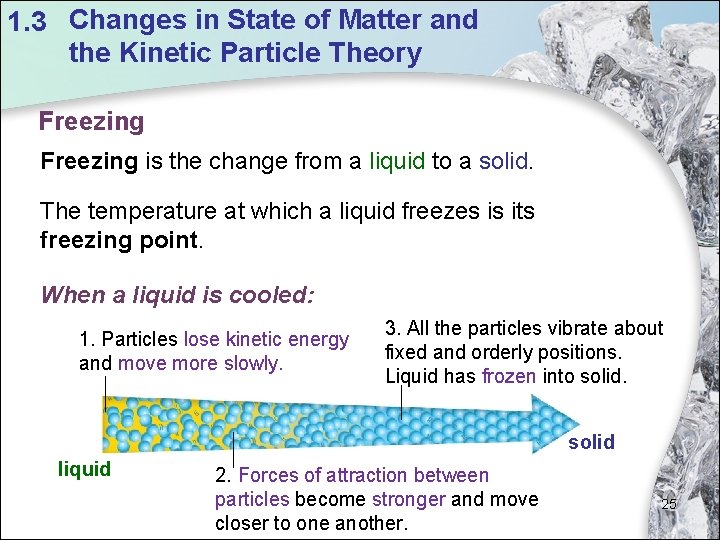
1. 3 Changes in State of Matter and the Kinetic Particle Theory Freezing is the change from a liquid to a solid. The temperature at which a liquid freezes is its freezing point. When a liquid is cooled: 1. Particles lose kinetic energy and move more slowly. 3. All the particles vibrate about fixed and orderly positions. Liquid has frozen into solid liquid 2. Forces of attraction between particles become stronger and move closer to one another. 25

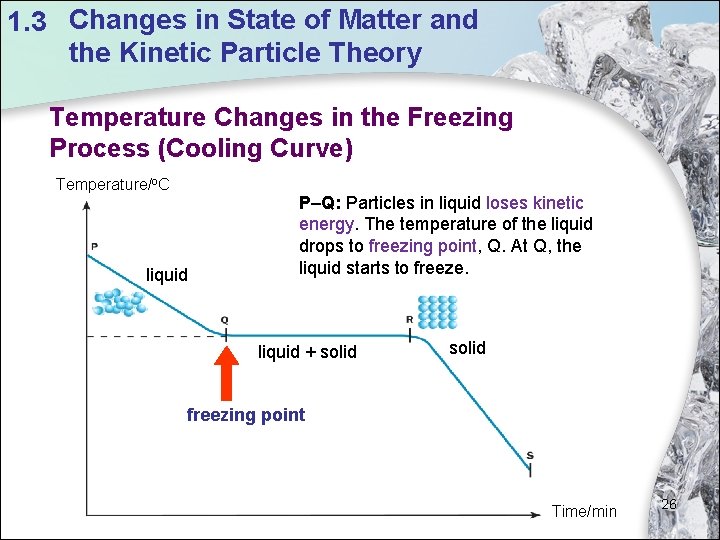
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Freezing Process (Cooling Curve) Temperature/o. C liquid P–Q: Particles in liquid loses kinetic energy. The temperature of the liquid drops to freezing point, Q. At Q, the liquid starts to freeze. liquid + solid freezing point Time/min 26

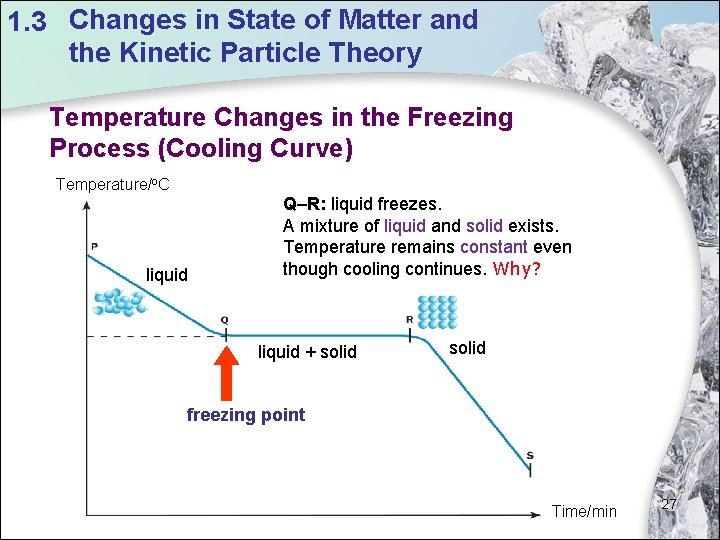
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Freezing Process (Cooling Curve) Temperature/o. C liquid Q–R: liquid freezes. A mixture of liquid and solid exists. Temperature remains constant even though cooling continues. Why? liquid + solid freezing point Time/min 27

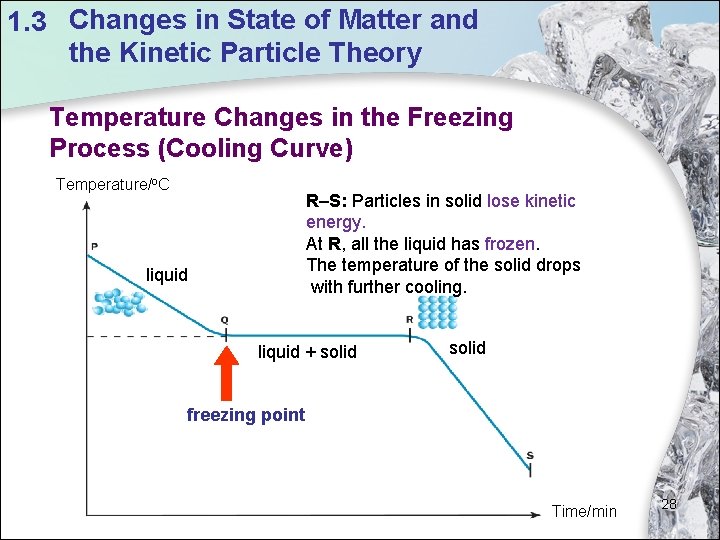
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Freezing Process (Cooling Curve) Temperature/o. C R–S: Particles in solid lose kinetic energy. At R, all the liquid has frozen. The temperature of the solid drops with further cooling. liquid + solid freezing point Time/min 28

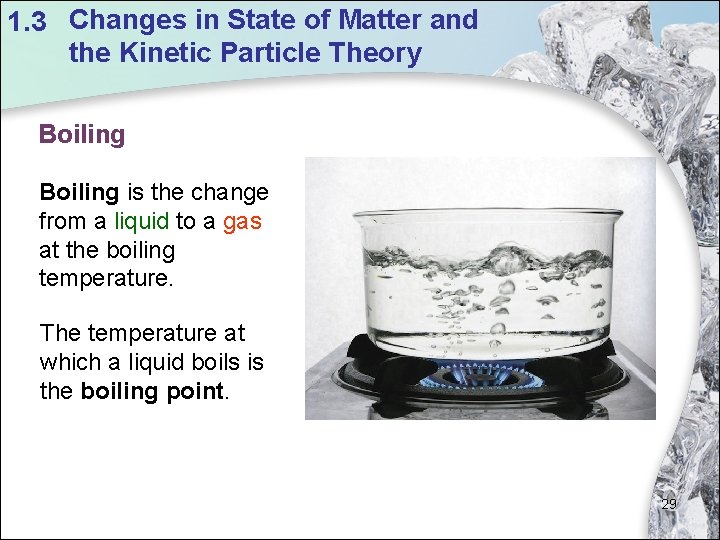
1. 3 Changes in State of Matter and the Kinetic Particle Theory Boiling is the change from a liquid to a gas at the boiling temperature. The temperature at which a liquid boils is the boiling point. 29

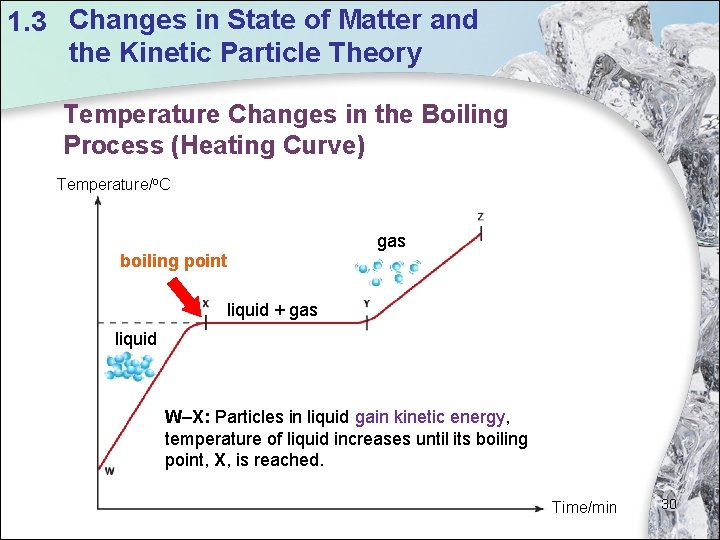
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Boiling Process (Heating Curve) Temperature/o. C gas boiling point liquid + gas liquid W–X: Particles in liquid gain kinetic energy, temperature of liquid increases until its boiling point, X, is reached. Time/min 30

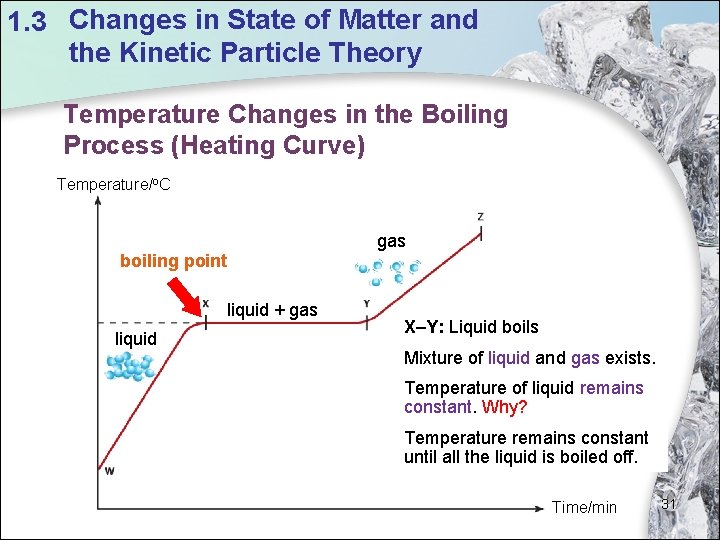
1. 3 Changes in State of Matter and the Kinetic Particle Theory Temperature Changes in the Boiling Process (Heating Curve) Temperature/o. C gas boiling point liquid + gas liquid X–Y: Liquid boils Mixture of liquid and gas exists. Temperature of liquid remains constant. Why? Temperature remains constant until all the liquid is boiled off. Time/min 31

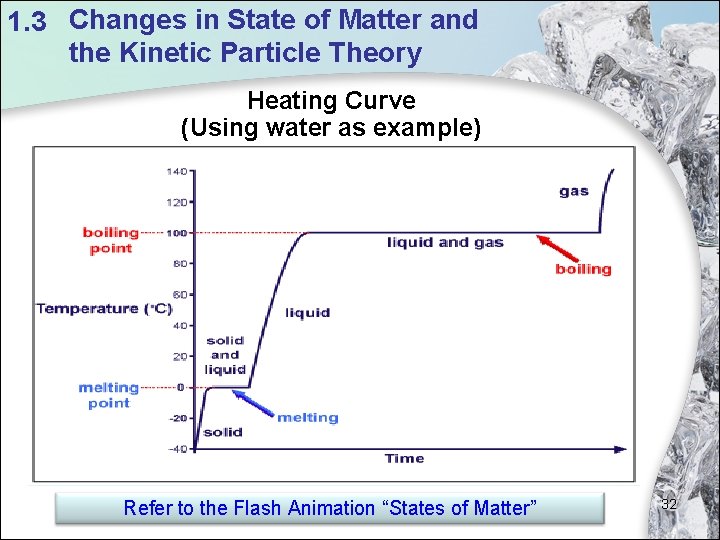
1. 3 Changes in State of Matter and the Kinetic Particle Theory Heating Curve (Using water as example) Refer to the Flash Animation “States of Matter” 32

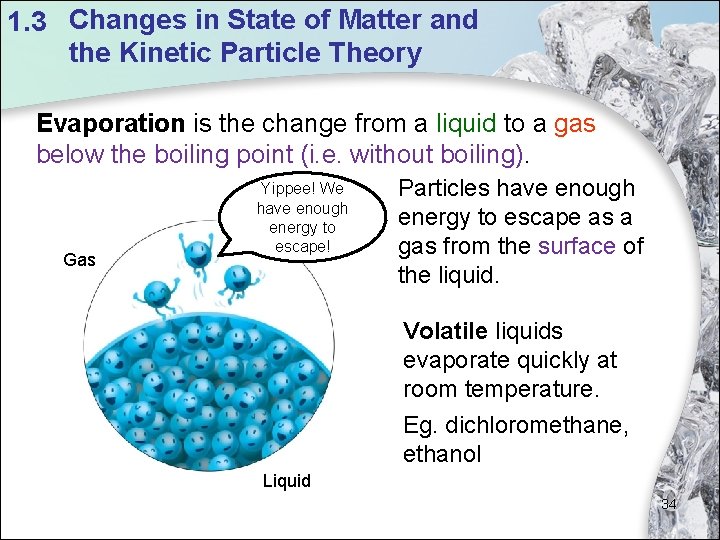
1. 3 Changes in State of Matter and the Kinetic Particle Theory What change of state is taking place here? Evaporation Clothes dry when water on wet clothes change into water vapour. 33

1. 3 Changes in State of Matter and the Kinetic Particle Theory Evaporation is the change from a liquid to a gas below the boiling point (i. e. without boiling). Gas Yippee! We have enough energy to escape! Particles have enough energy to escape as a gas from the surface of the liquid. Volatile liquids evaporate quickly at room temperature. Eg. dichloromethane, ethanol Liquid 34

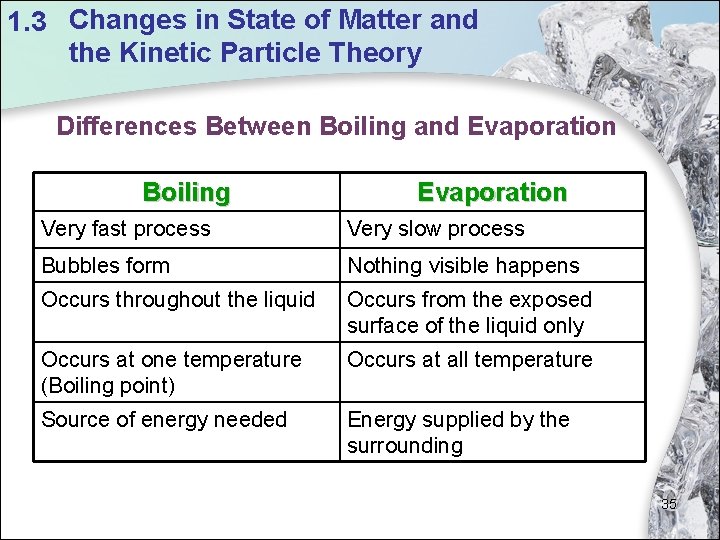
1. 3 Changes in State of Matter and the Kinetic Particle Theory Differences Between Boiling and Evaporation Boiling Evaporation Very fast process Very slow process Bubbles form Nothing visible happens Occurs throughout the liquid Occurs from the exposed surface of the liquid only Occurs at one temperature (Boiling point) Occurs at all temperature Source of energy needed Energy supplied by the surrounding 35

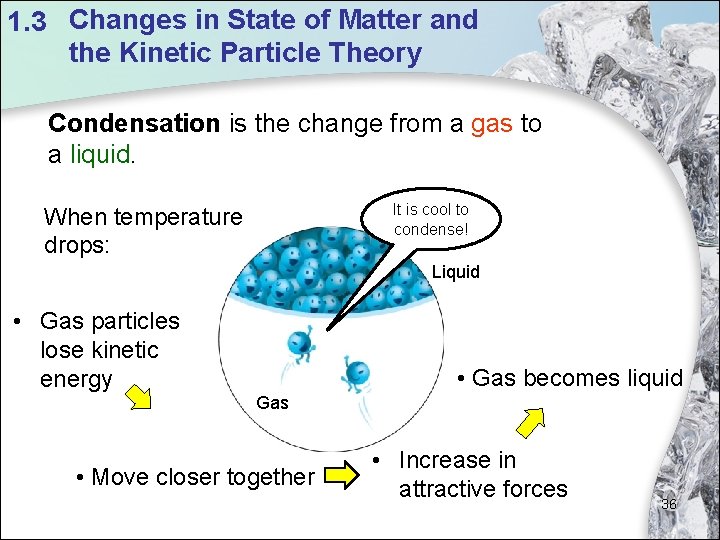
1. 3 Changes in State of Matter and the Kinetic Particle Theory Condensation is the change from a gas to a liquid. It is cool to condense! When temperature drops: Liquid • Gas particles lose kinetic energy • Gas becomes liquid Gas • Move closer together • Increase in attractive forces 36

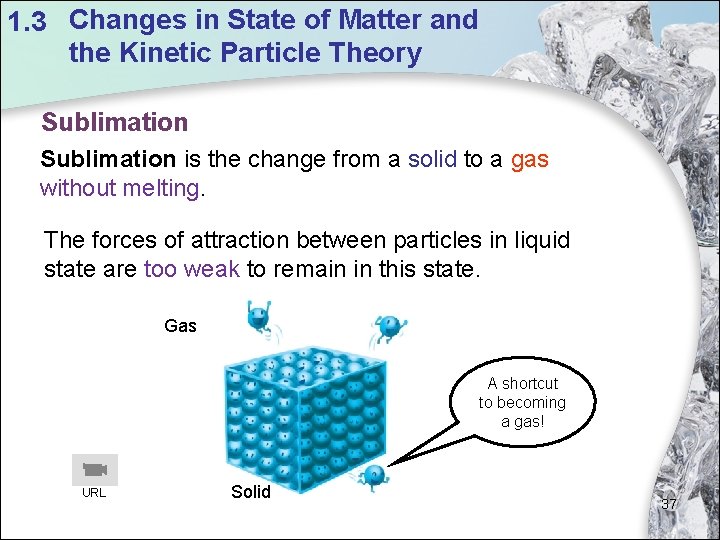
1. 3 Changes in State of Matter and the Kinetic Particle Theory Sublimation is the change from a solid to a gas without melting. The forces of attraction between particles in liquid state are too weak to remain in this state. Gas A shortcut to becoming a gas! URL Solid 37

Chapter 1 Kinetic Particle Theory 1. 1 States of Matter 1. 2 Kinetic Particle Theory 1. 3 Changes in State of Matter and the Kinetic Particle Theory 1. 4 Diffusion 38

1. 4 Diffusion Learning Outcomes At the end of this section, you should be able to: • use diffusion to account for the movement of particles in liquids and gases; • explain daily effects of diffusion in terms of particles; • state qualitatively the effect of molecular mass on the rate of diffusion and explain the dependence of rate of diffusion on temperature 39

1. 4 Diffusion Some Examples… • When a bottle of perfume is left open for some time, the scent of the perfume soon spreads throughout the entire room. • Similarly, if your mother is cooking curry in the kitchen, you will soon be able to detect the smell of spices in every room. • Diffusion is an evidence of the kinetic particle theory. 40

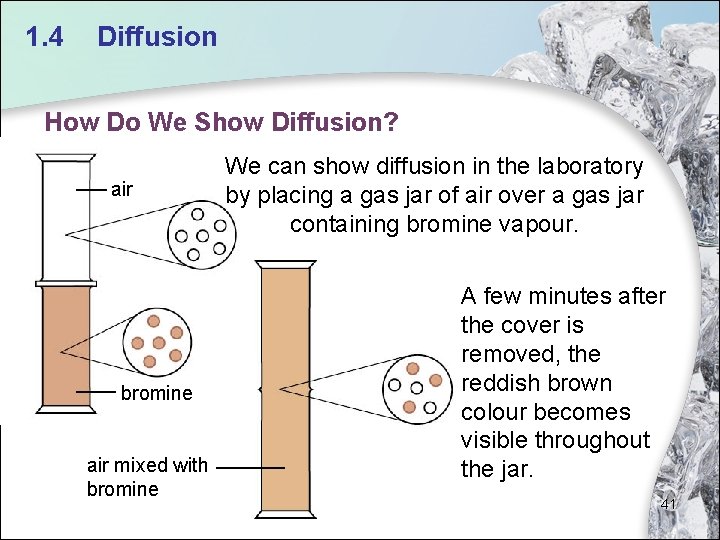
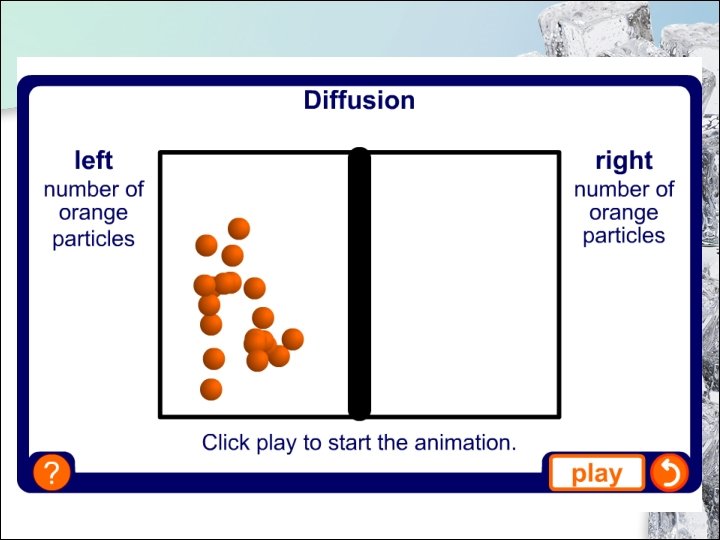
1. 4 Diffusion How Do We Show Diffusion? air bromine air mixed with bromine We can show diffusion in the laboratory by placing a gas jar of air over a gas jar containing bromine vapour. A few minutes after the cover is removed, the reddish brown colour becomes visible throughout the jar. 41

How Do We Show Diffusion? Reddish brown colour becomes visible throughout the jar. 42

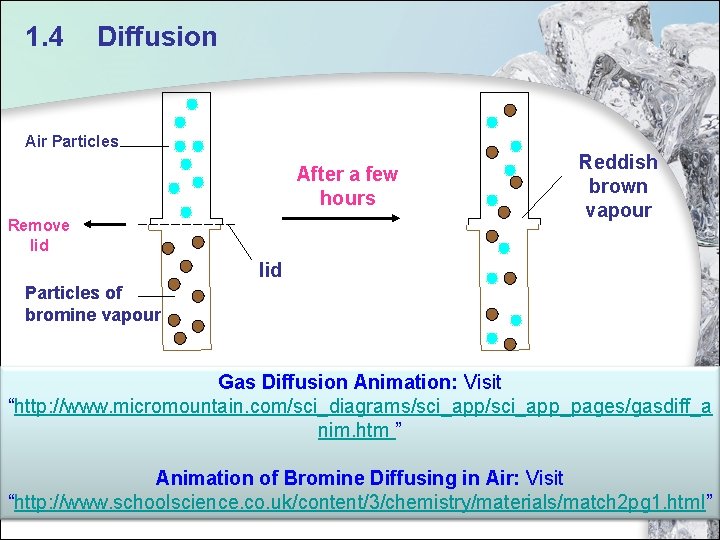
1. 4 Diffusion Air Particles After a few hours Remove lid Reddish brown vapour lid Particles of bromine vapour Gas Diffusion Animation: Visit “http: //www. micromountain. com/sci_diagrams/sci_app_pages/gasdiff_a nim. htm ” Animation of Bromine Diffusing in Air: Visit 43 “http: //www. schoolscience. co. uk/content/3/chemistry/materials/match 2 pg 1. html”

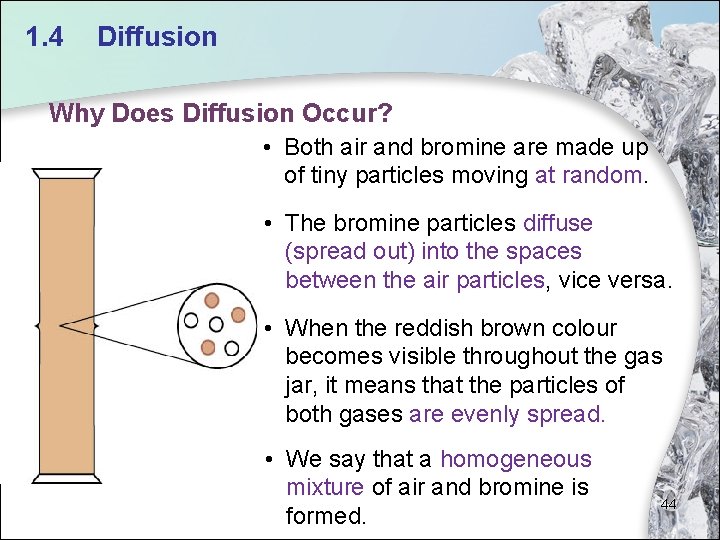
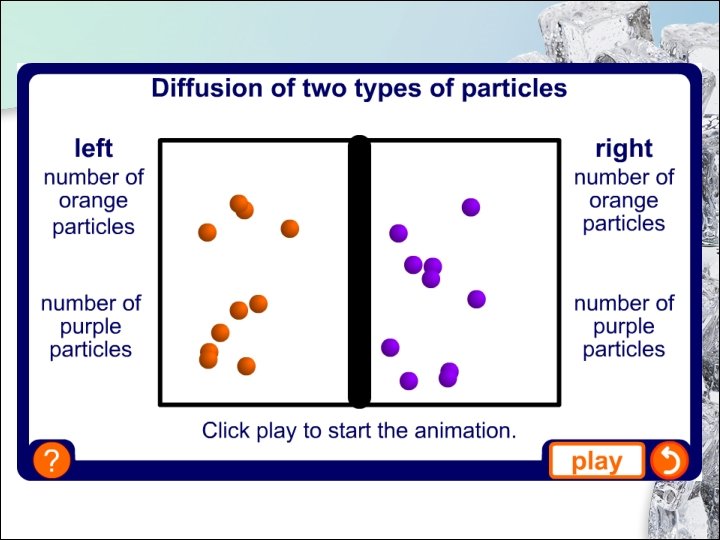
1. 4 Diffusion Why Does Diffusion Occur? • Both air and bromine are made up of tiny particles moving at random. • The bromine particles diffuse (spread out) into the spaces between the air particles, vice versa. • When the reddish brown colour becomes visible throughout the gas jar, it means that the particles of both gases are evenly spread. • We say that a homogeneous mixture of air and bromine is formed. 44

1. 4 Diffusion Why Does Diffusion Occur? Diffusion is • the movement of particles from a region of higher concentration to a region of lower concentration due to constant and random motion of the particles. • This is a process by which particles move freely to fill up any available space. 45



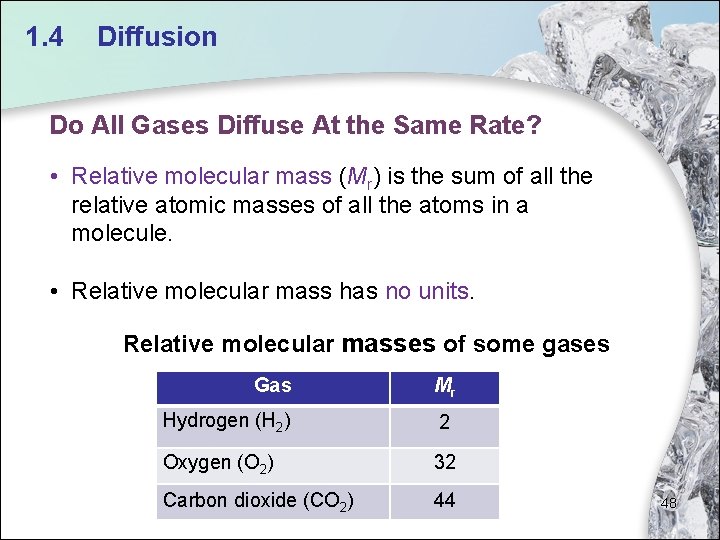
1. 4 Diffusion Do All Gases Diffuse At the Same Rate? • Relative molecular mass (Mr) is the sum of all the relative atomic masses of all the atoms in a molecule. • Relative molecular mass has no units. Relative molecular masses of some gases Gas Mr Hydrogen (H 2) 2 Oxygen (O 2) 32 Carbon dioxide (CO 2) 44 48

1. 4 Diffusion Do All Gases Diffuse At the Same Rate? • Gas particles diffuse at different speeds depending on their relative molecular mass. • Under the same conditions of temperature and pressure, a gas with a lower relative molecular mass diffuse faster than a gas with a higher relative molecular mass. Gases with lower relative molecular masses diffuse faster than those with higher relative molecular masses. 49

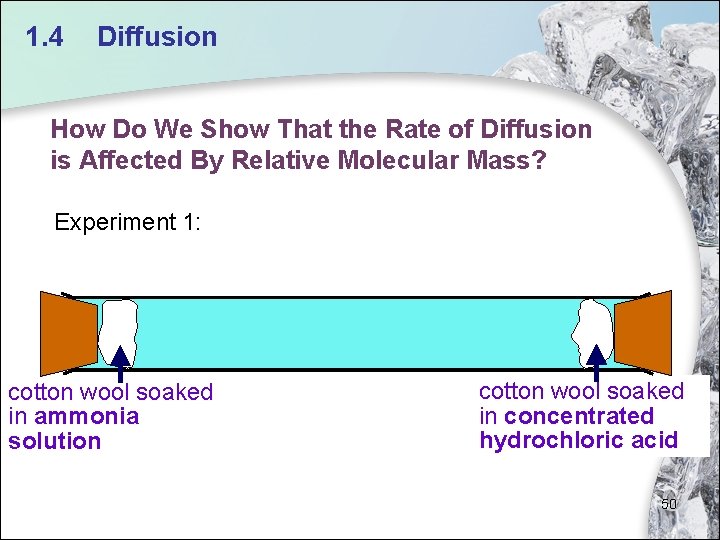
1. 4 Diffusion How Do We Show That the Rate of Diffusion is Affected By Relative Molecular Mass? Experiment 1: cotton wool soaked in ammonia solution cotton wool soaked in concentrated hydrochloric acid 50

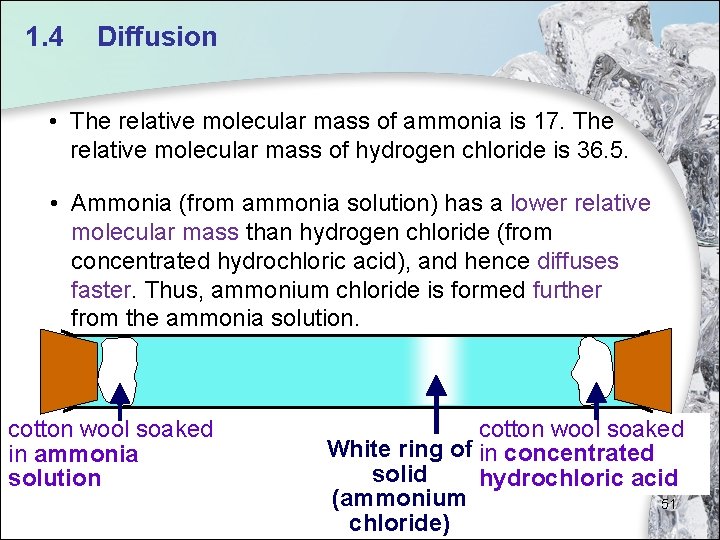
1. 4 Diffusion • The relative molecular mass of ammonia is 17. The relative molecular mass of hydrogen chloride is 36. 5. • Ammonia (from ammonia solution) has a lower relative molecular mass than hydrogen chloride (from concentrated hydrochloric acid), and hence diffuses faster. Thus, ammonium chloride is formed further from the ammonia solution. cotton wool soaked in ammonia solution cotton wool soaked White ring of in concentrated solid hydrochloric acid (ammonium 51 chloride)

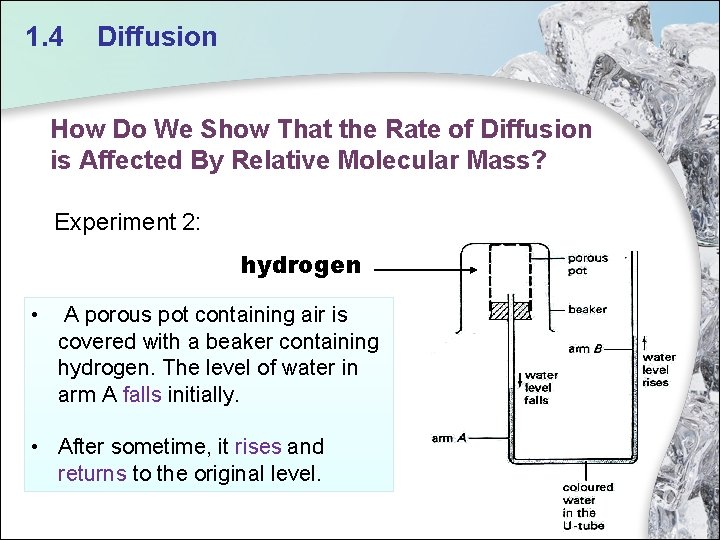
1. 4 Diffusion How Do We Show That the Rate of Diffusion is Affected By Relative Molecular Mass? Experiment 2: hydrogen • A porous pot containing air is covered with a beaker containing hydrogen. The level of water in arm A falls initially. • After sometime, it rises and returns to the original level.

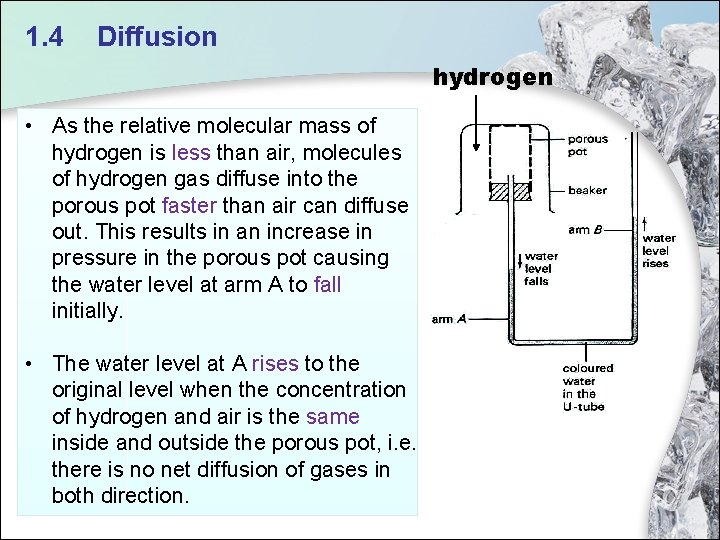
1. 4 Diffusion hydrogen • As the relative molecular mass of hydrogen is less than air, molecules of hydrogen gas diffuse into the porous pot faster than air can diffuse out. This results in an increase in pressure in the porous pot causing the water level at arm A to fall initially. • The water level at A rises to the original level when the concentration of hydrogen and air is the same inside and outside the porous pot, i. e. there is no net diffusion of gases in both direction.

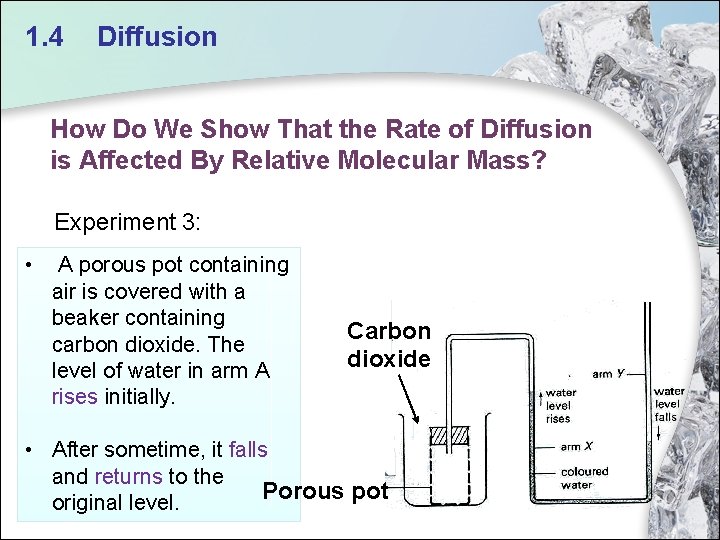
1. 4 Diffusion How Do We Show That the Rate of Diffusion is Affected By Relative Molecular Mass? Experiment 3: • A porous pot containing air is covered with a beaker containing carbon dioxide. The level of water in arm A rises initially. Carbon dioxide • After sometime, it falls and returns to the Porous pot original level.

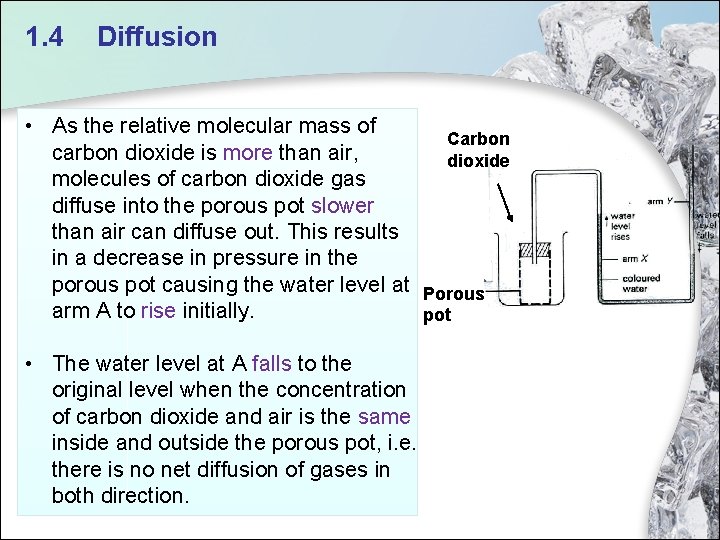
1. 4 Diffusion • As the relative molecular mass of carbon dioxide is more than air, molecules of carbon dioxide gas diffuse into the porous pot slower than air can diffuse out. This results in a decrease in pressure in the porous pot causing the water level at arm A to rise initially. • The water level at A falls to the original level when the concentration of carbon dioxide and air is the same inside and outside the porous pot, i. e. there is no net diffusion of gases in both direction. Carbon dioxide Porous pot

1. 4 Diffusion in Liquids Diffusion also takes place in liquids. • A small crystal of potassium manganate(VII) is introduced into a beaker of distilled water. A deep purple solution forms at the bottom of the beaker. • Diffusion slowly takes place until the solution becomes uniformly purple. 56

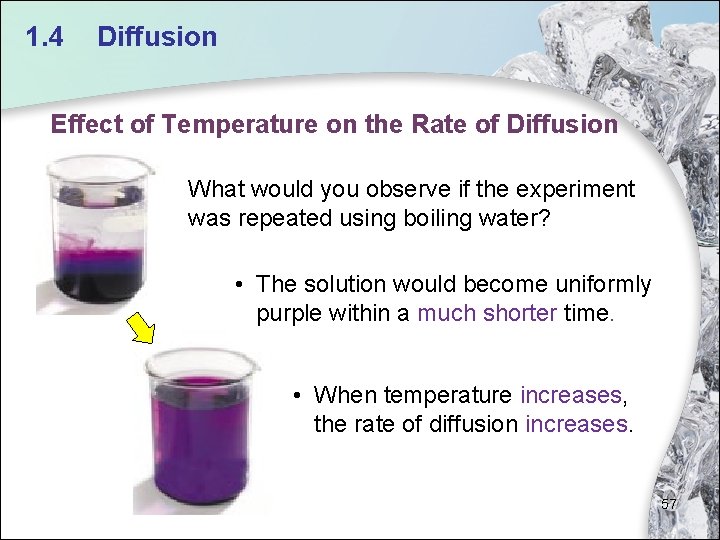
1. 4 Diffusion Effect of Temperature on the Rate of Diffusion What would you observe if the experiment was repeated using boiling water? • The solution would become uniformly purple within a much shorter time. • When temperature increases, the rate of diffusion increases. 57

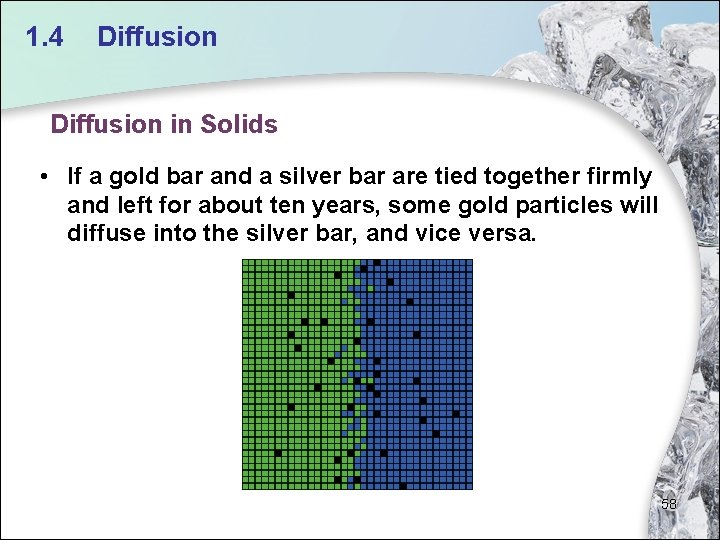
1. 4 Diffusion in Solids • If a gold bar and a silver bar are tied together firmly and left for about ten years, some gold particles will diffuse into the silver bar, and vice versa. 58

Diffusion is fastest in gases, where the particles are far apart and the attractive forces between particles are negligible. It is the slowest in solids, where the particles are held closely together in fixed positions by strong attractive forces. 59

Anagrams