Chapter 1 IronCarbon Alloys MS 371 Structure and

Chapter 1 Iron-Carbon AlloysⅠ /MS 371/ Structure and Properties of Engineering Alloys
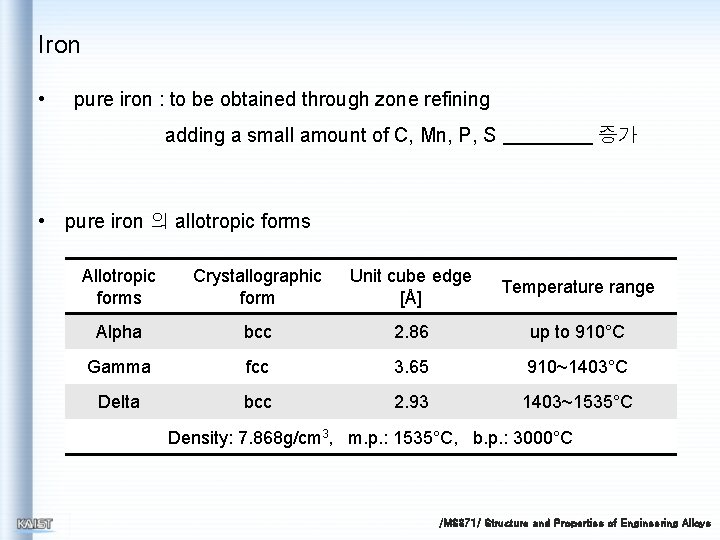
Iron • pure iron : to be obtained through zone refining adding a small amount of C, Mn, P, S 증가 • pure iron 의 allotropic forms Allotropic forms Crystallographic form Unit cube edge [Å] Temperature range Alpha bcc 2. 86 up to 910°C Gamma fcc 3. 65 910~1403°C Delta bcc 2. 93 1403~1535°C Density: 7. 868 g/cm 3, m. p. : 1535°C, b. p. : 3000°C /MS 371/ Structure and Properties of Engineering Alloys
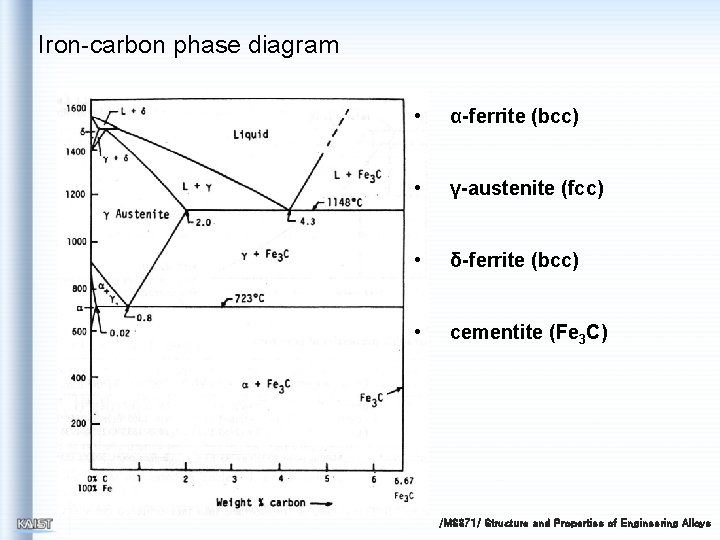
Iron-carbon phase diagram • α-ferrite (bcc) • γ-austenite (fcc) • δ-ferrite (bcc) • cementite (Fe 3 C) /MS 371/ Structure and Properties of Engineering Alloys
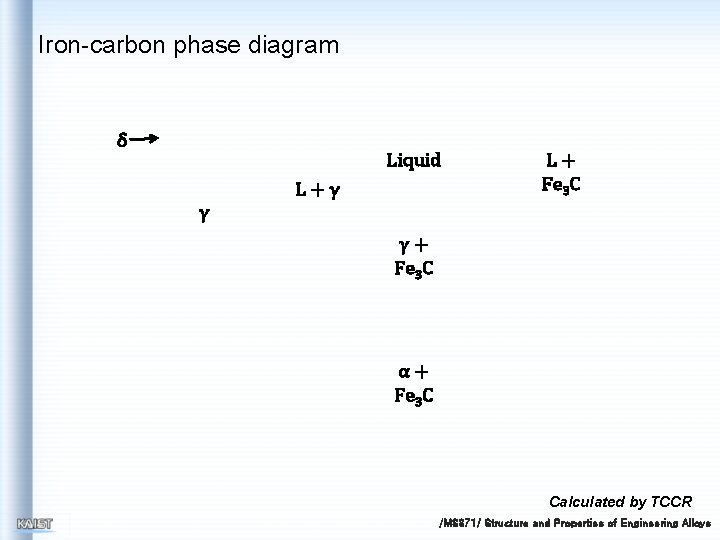
Iron-carbon phase diagram δ Liquid L+γ L+ Fe 3 C γ γ+ Fe 3 C α+ Fe 3 C Calculated by TCCR /MS 371/ Structure and Properties of Engineering Alloys
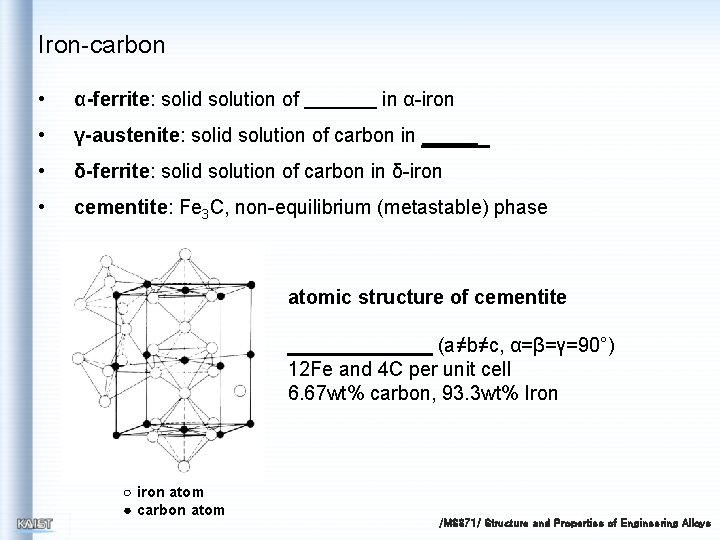
Iron-carbon • α-ferrite: solid solution of • γ-austenite: solid solution of carbon in • δ-ferrite: solid solution of carbon in δ-iron • cementite: Fe 3 C, non-equilibrium (metastable) phase in α-iron atomic structure of cementite (a≠b≠c, α=β=γ=90°) 12 Fe and 4 C per unit cell 6. 67 wt% carbon, 93. 3 wt% Iron ○ iron atom ● carbon atom /MS 371/ Structure and Properties of Engineering Alloys
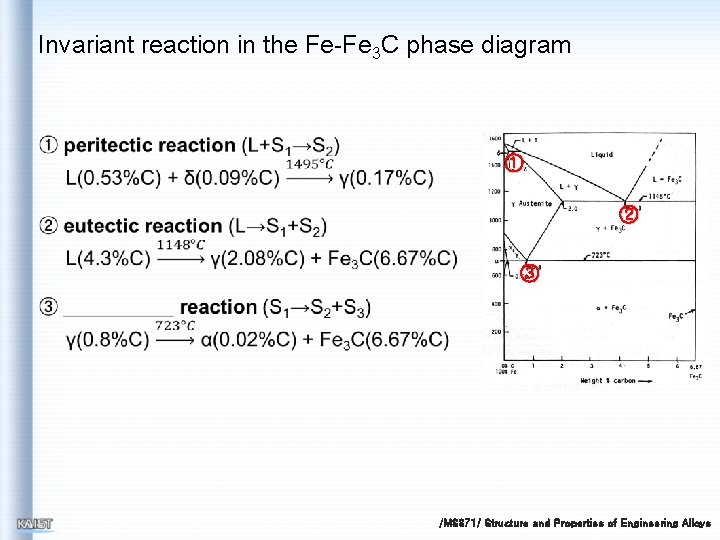
Invariant reaction in the Fe-Fe 3 C phase diagram ① ② ③ /MS 371/ Structure and Properties of Engineering Alloys
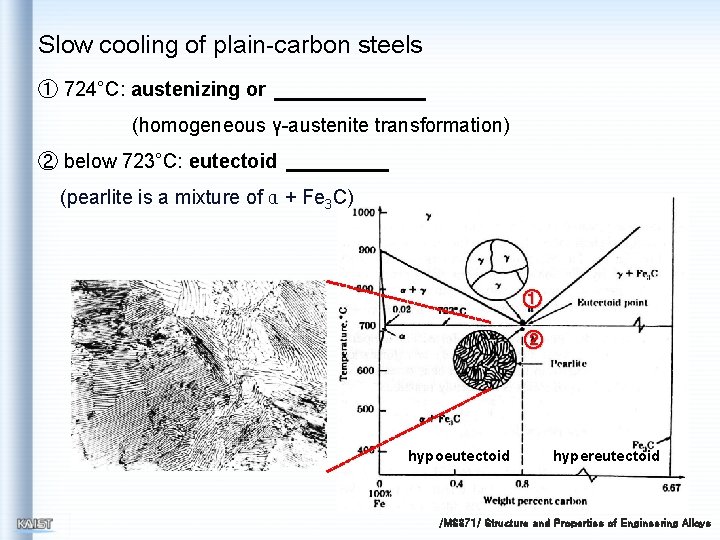
Slow cooling of plain-carbon steels ① 724°C: austenizing or (homogeneous γ-austenite transformation) ② below 723°C: eutectoid (pearlite is a mixture of α + Fe 3 C) ① ② hypoeutectoid hypereutectoid /MS 371/ Structure and Properties of Engineering Alloys
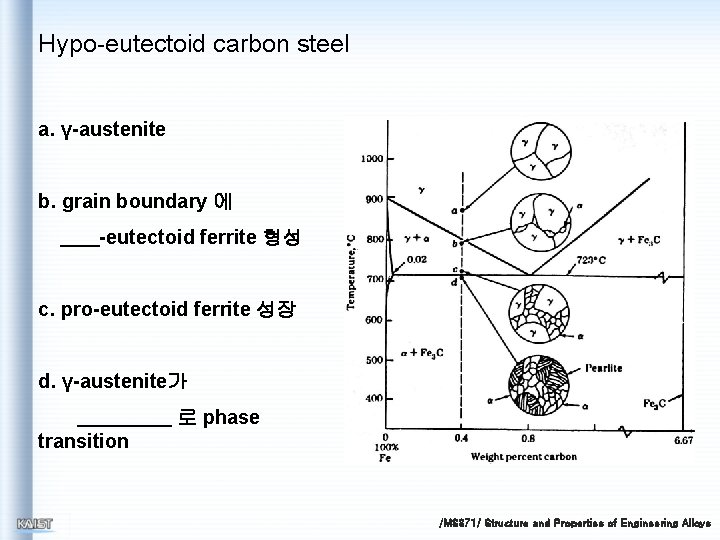
Hypo-eutectoid carbon steel a. γ-austenite b. grain boundary 에 -eutectoid ferrite 형성 c. pro-eutectoid ferrite 성장 d. γ-austenite가 로 phase transition /MS 371/ Structure and Properties of Engineering Alloys
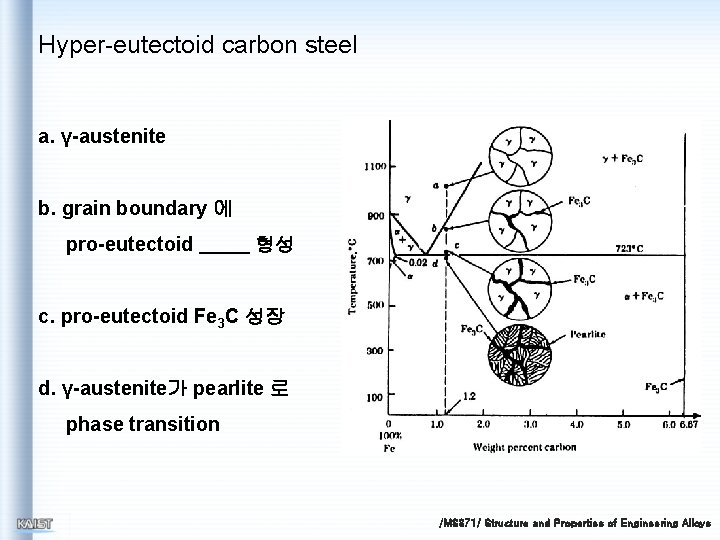
Hyper-eutectoid carbon steel a. γ-austenite b. grain boundary 에 pro-eutectoid 형성 c. pro-eutectoid Fe 3 C 성장 d. γ-austenite가 pearlite 로 phase transition /MS 371/ Structure and Properties of Engineering Alloys
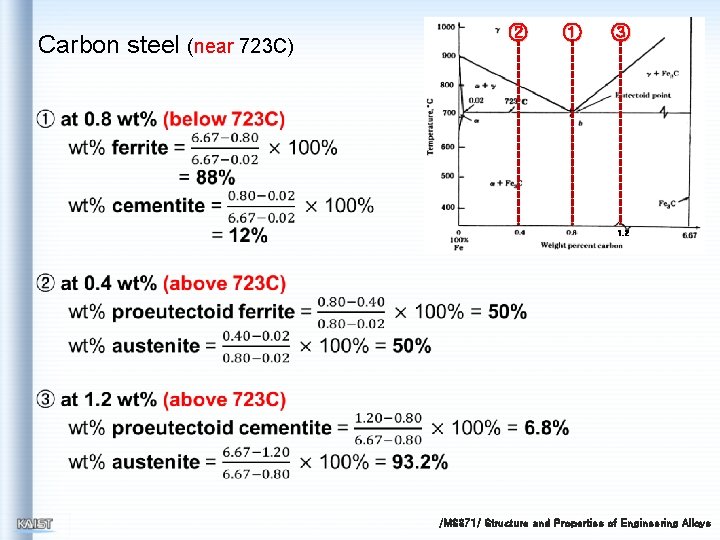
Carbon steel (near 723 C) ② ① ③ 1. 2 /MS 371/ Structure and Properties of Engineering Alloys
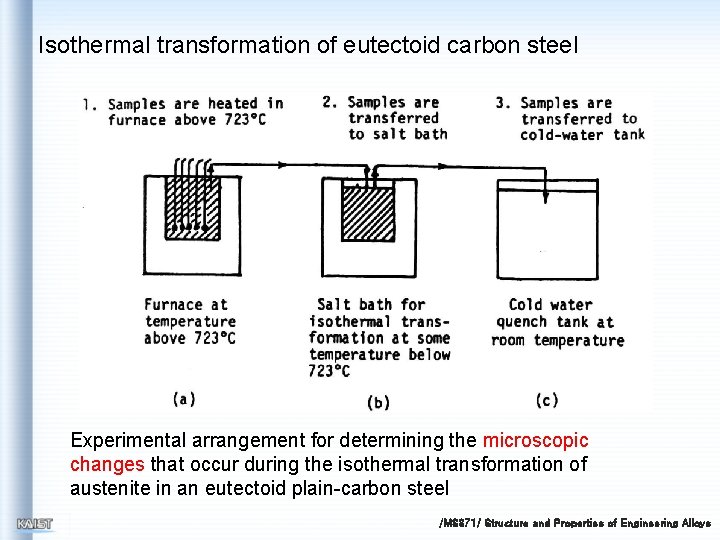
Isothermal transformation of eutectoid carbon steel Experimental arrangement for determining the microscopic changes that occur during the isothermal transformation of austenite in an eutectoid plain-carbon steel /MS 371/ Structure and Properties of Engineering Alloys
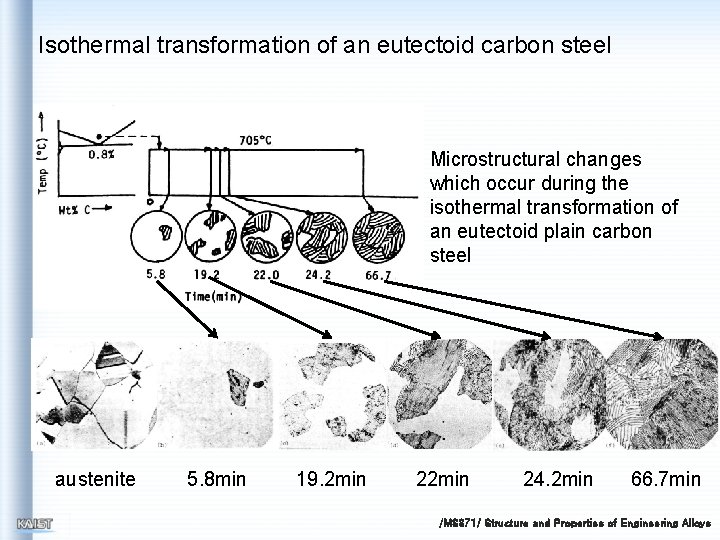
Isothermal transformation of an eutectoid carbon steel Microstructural changes which occur during the isothermal transformation of an eutectoid plain carbon steel austenite 5. 8 min 19. 2 min 24. 2 min 66. 7 min /MS 371/ Structure and Properties of Engineering Alloys
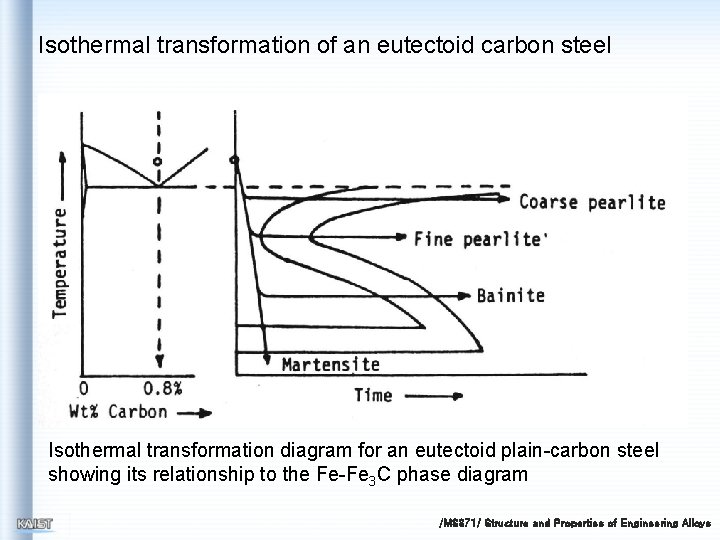
Isothermal transformation of an eutectoid carbon steel Isothermal transformation diagram for an eutectoid plain-carbon steel showing its relationship to the Fe-Fe 3 C phase diagram /MS 371/ Structure and Properties of Engineering Alloys
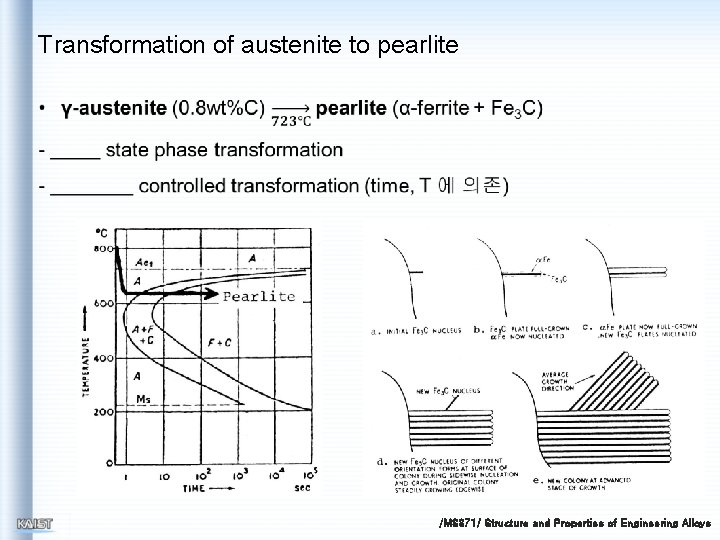
Transformation of austenite to pearlite /MS 371/ Structure and Properties of Engineering Alloys
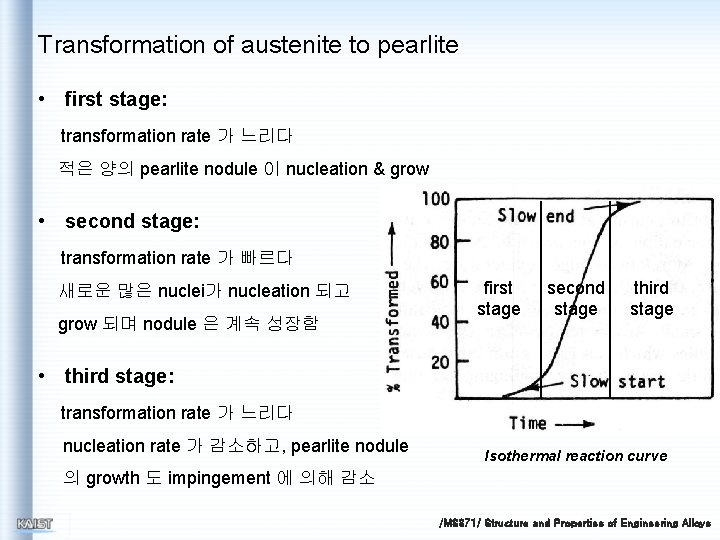
Transformation of austenite to pearlite • first stage: transformation rate 가 느리다 적은 양의 pearlite nodule 이 nucleation & grow • second stage: transformation rate 가 빠르다 새로운 많은 nuclei가 nucleation 되고 grow 되며 nodule 은 계속 성장함 first stage second stage third stage • third stage: transformation rate 가 느리다 nucleation rate 가 감소하고, pearlite nodule Isothermal reaction curve 의 growth 도 impingement 에 의해 감소 /MS 371/ Structure and Properties of Engineering Alloys
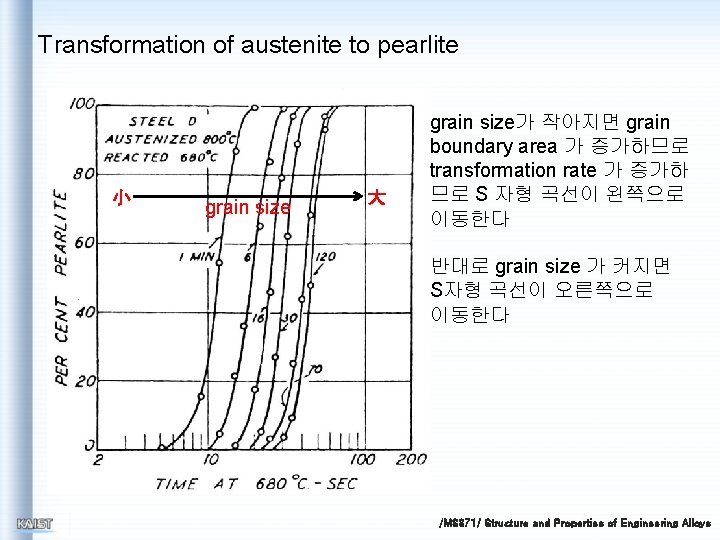
Transformation of austenite to pearlite 小 grain size 大 grain size가 작아지면 grain boundary area 가 증가하므로 transformation rate 가 증가하 므로 S 자형 곡선이 왼쪽으로 이동한다 반대로 grain size 가 커지면 S자형 곡선이 오른쪽으로 이동한다 /MS 371/ Structure and Properties of Engineering Alloys
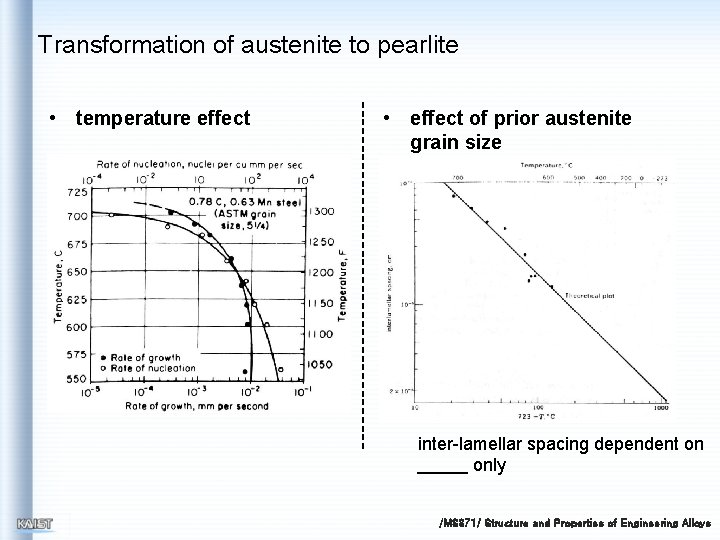
Transformation of austenite to pearlite • temperature effect • effect of prior austenite grain size inter-lamellar spacing dependent on only /MS 371/ Structure and Properties of Engineering Alloys
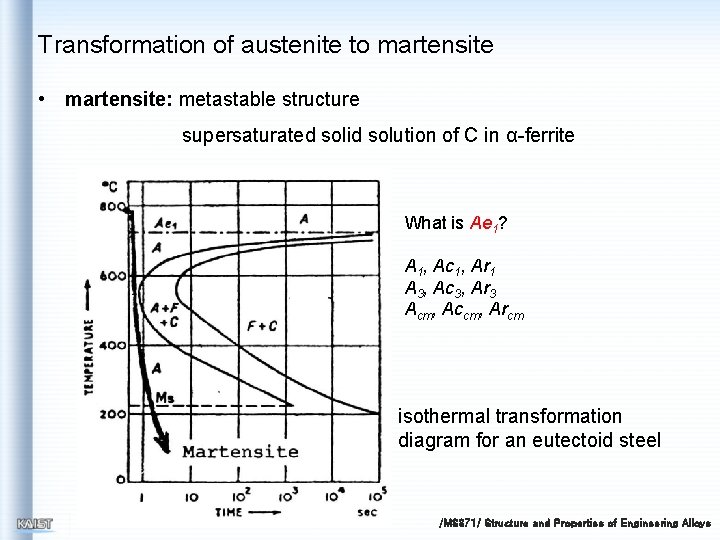
Transformation of austenite to martensite • martensite: metastable structure supersaturated solid solution of C in α-ferrite What is Ae 1? A 1, Ac 1, Ar 1 A 3, Ac 3, Ar 3 Acm, Accm, Arcm isothermal transformation diagram for an eutectoid steel /MS 371/ Structure and Properties of Engineering Alloys
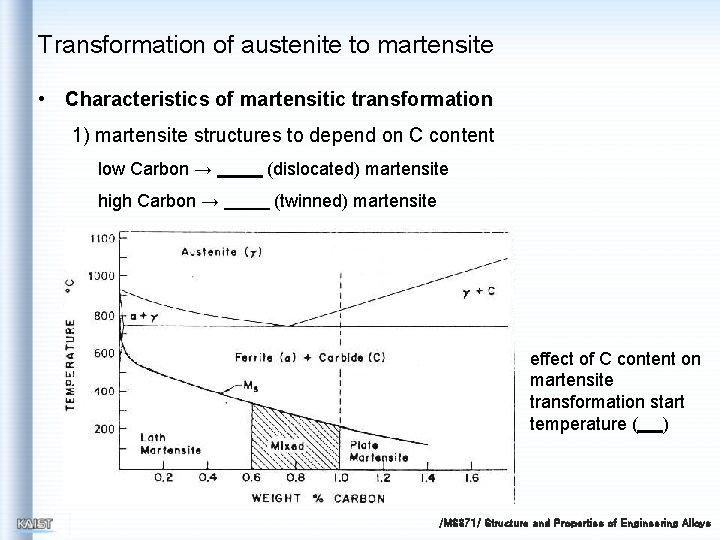
Transformation of austenite to martensite • Characteristics of martensitic transformation 1) martensite structures to depend on C content low Carbon → (dislocated) martensite high Carbon → (twinned) martensite effect of C content on martensite transformation start temperature ( ) /MS 371/ Structure and Properties of Engineering Alloys
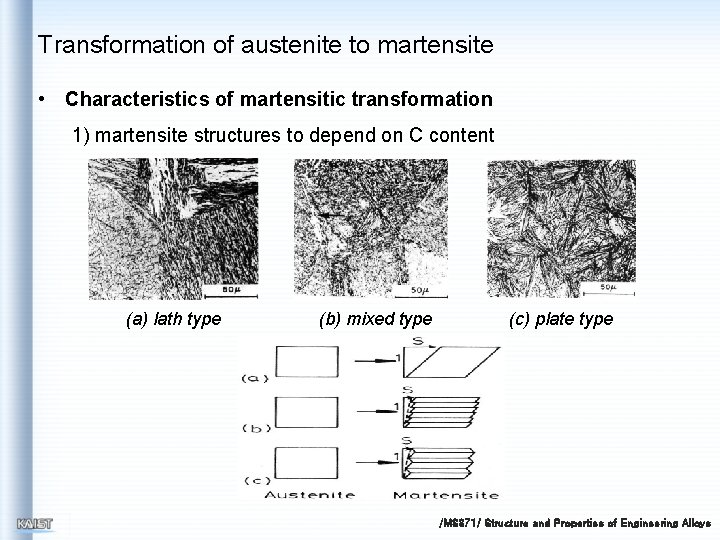
Transformation of austenite to martensite • Characteristics of martensitic transformation 1) martensite structures to depend on C content (a) lath type (b) mixed type (c) plate type /MS 371/ Structure and Properties of Engineering Alloys
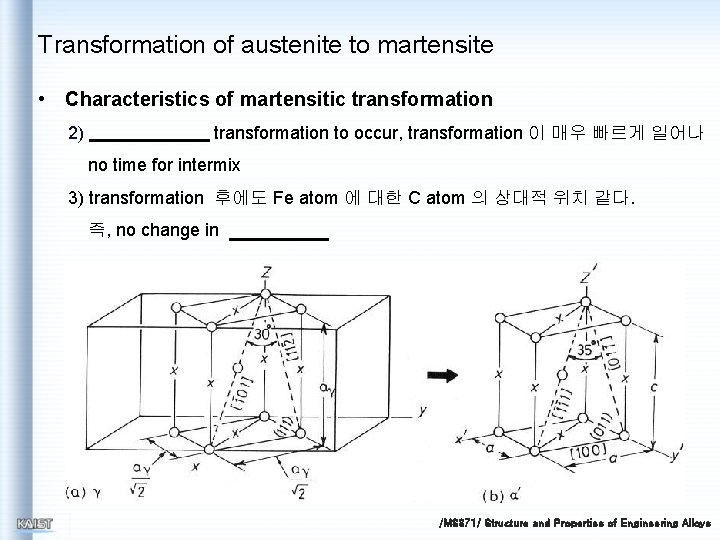
Transformation of austenite to martensite • Characteristics of martensitic transformation 2) transformation to occur, transformation 이 매우 빠르게 일어나 no time for intermix 3) transformation 후에도 Fe atom 에 대한 C atom 의 상대적 위치 같다. 즉, no change in /MS 371/ Structure and Properties of Engineering Alloys
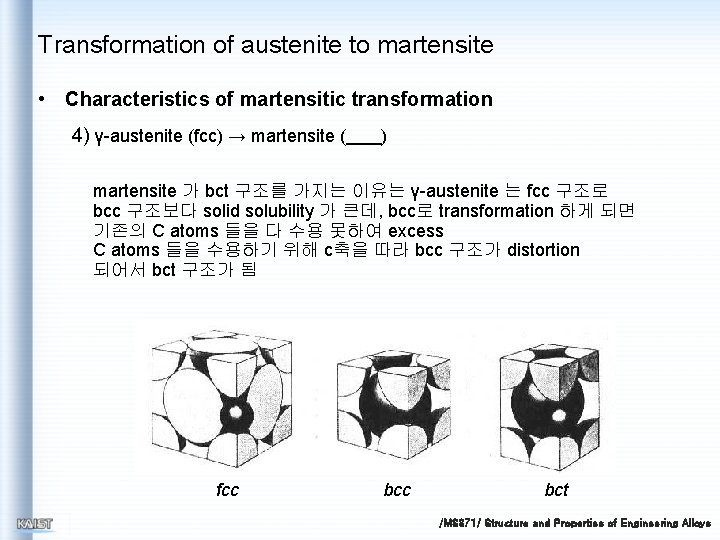
Transformation of austenite to martensite • Characteristics of martensitic transformation 4) γ-austenite (fcc) → martensite ( ) martensite 가 bct 구조를 가지는 이유는 γ-austenite 는 fcc 구조로 bcc 구조보다 solid solubility 가 큰데, bcc로 transformation 하게 되면 기존의 C atoms 들을 다 수용 못하여 excess C atoms 들을 수용하기 위해 c축을 따라 bcc 구조가 distortion 되어서 bct 구조가 됨 fcc bct /MS 371/ Structure and Properties of Engineering Alloys
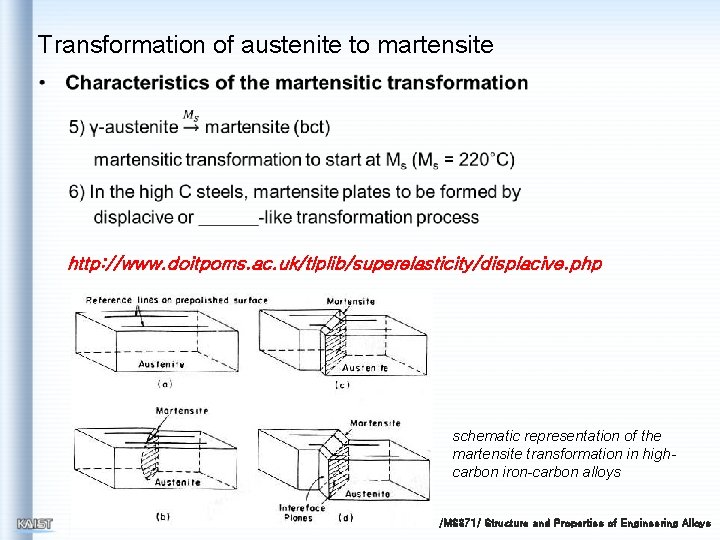
Transformation of austenite to martensite http: //www. doitpoms. ac. uk/tlplib/superelasticity/displacive. php schematic representation of the martensite transformation in highcarbon iron-carbon alloys /MS 371/ Structure and Properties of Engineering Alloys
Transformation of austenite to martensite • Morphology of martensite in Fe-C alloys lath martensite: dislocation martensite (slip이 발생) domain 내의 각각의 lath 들은 일정한 orientation 을 가짐 lath 들은 highly distortion 되어 있고 높은 밀도의 dislocation 들이 tangle 된 지역을 이루고 있음 almost no retained γ-austenite plate martensite: needle-like plate 들이 habit plane {225}에서 {259} 까지 위에서 independent 하게 형성됨 dislocation density 가 낮음 plate 의 크기는 다양하고 {112} 위에 twin 들이 존재 lath and plate (mixed) martensite: 탄소 함량 0. 6~1. 0%, 온도 범위 200~320°C /MS 371/ Structure and Properties of Engineering Alloys
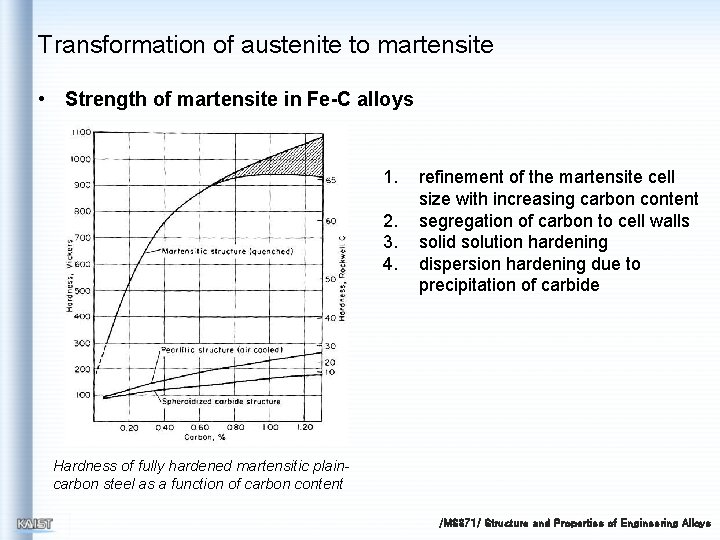
Transformation of austenite to martensite • Strength of martensite in Fe-C alloys 1. 2. 3. 4. refinement of the martensite cell size with increasing carbon content segregation of carbon to cell walls solid solution hardening dispersion hardening due to precipitation of carbide Hardness of fully hardened martensitic plaincarbon steel as a function of carbon content /MS 371/ Structure and Properties of Engineering Alloys
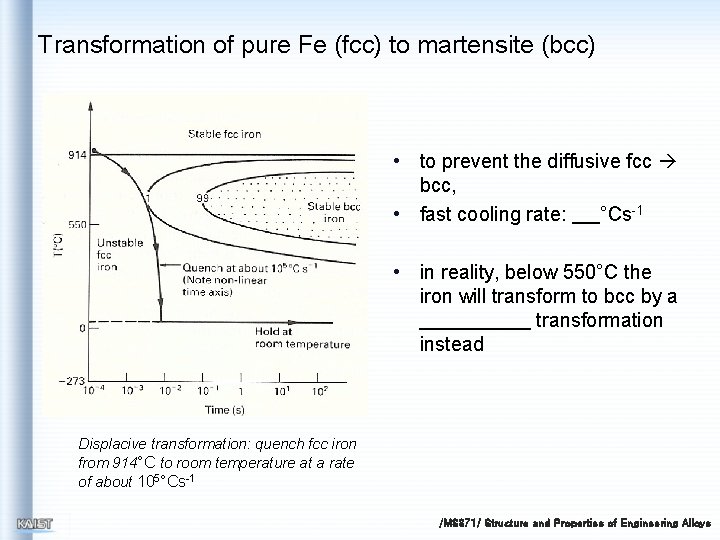
Transformation of pure Fe (fcc) to martensite (bcc) • to prevent the diffusive fcc bcc, • fast cooling rate: °Cs-1 • in reality, below 550°C the iron will transform to bcc by a transformation instead Displacive transformation: quench fcc iron from 914°C to room temperature at a rate of about 105°Cs-1 /MS 371/ Structure and Properties of Engineering Alloys
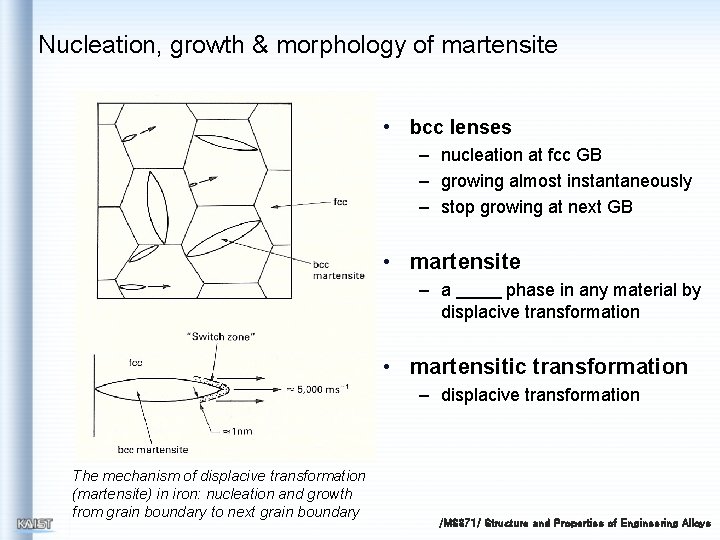
Nucleation, growth & morphology of martensite • bcc lenses – nucleation at fcc GB – growing almost instantaneously – stop growing at next GB • martensite – a phase in any material by displacive transformation • martensitic transformation – displacive transformation The mechanism of displacive transformation (martensite) in iron: nucleation and growth from grain boundary to next grain boundary /MS 371/ Structure and Properties of Engineering Alloys
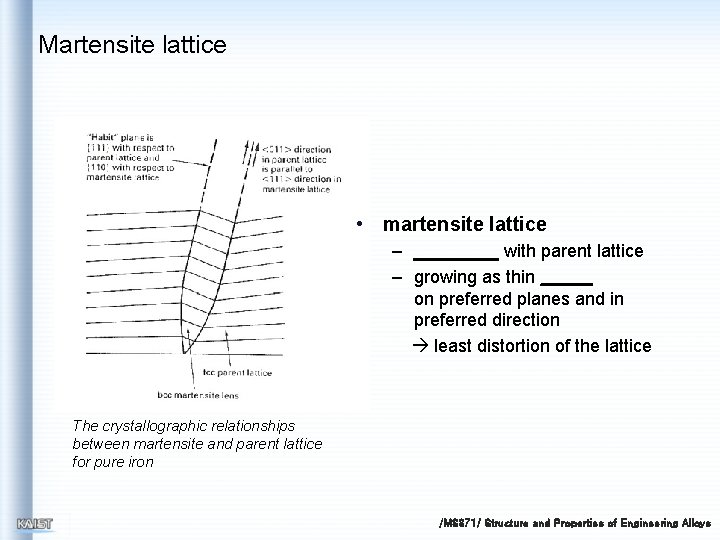
Martensite lattice • martensite lattice – with parent lattice – growing as thin on preferred planes and in preferred direction least distortion of the lattice The crystallographic relationships between martensite and parent lattice for pure iron /MS 371/ Structure and Properties of Engineering Alloys
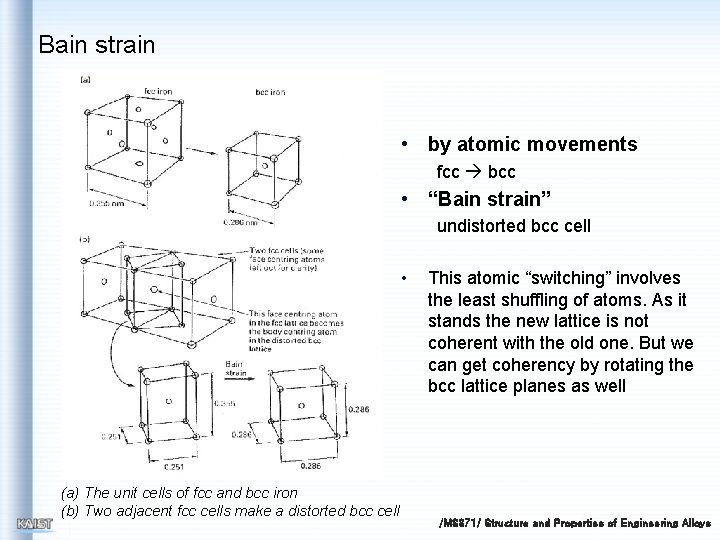
Bain strain • by atomic movements fcc bcc • “Bain strain” undistorted bcc cell • (a) The unit cells of fcc and bcc iron (b) Two adjacent fcc cells make a distorted bcc cell This atomic “switching” involves the least shuffling of atoms. As it stands the new lattice is not coherent with the old one. But we can get coherency by rotating the bcc lattice planes as well /MS 371/ Structure and Properties of Engineering Alloys
- Slides: 29