CHAPTER 1 Introduction To Science 1 1 Science






















- Slides: 29

CHAPTER 1 Introduction To Science

1. 1 Science is Part of everyday life

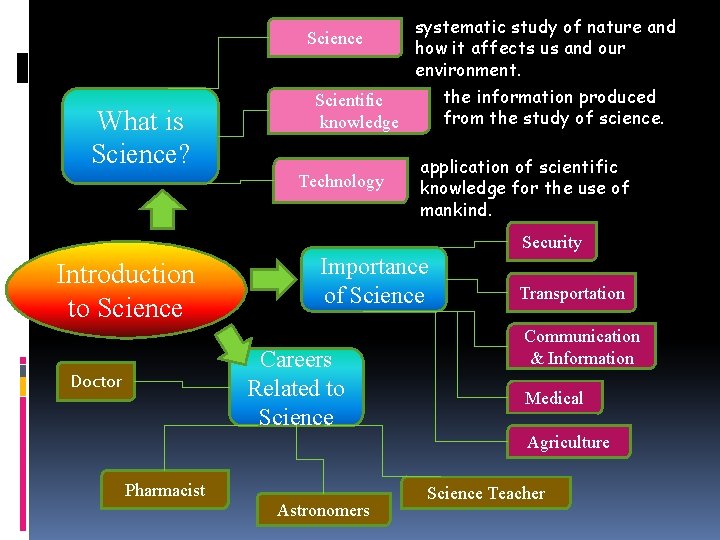
systematic study of nature and how it affects us and our environment. the information produced Scientific from the study of science. knowledge Science What is Science? Technology application of scientific knowledge for the use of mankind. Security Introduction to Science Importance of Science Careers Related to Science Doctor Transportation Communication & Information Medical Agriculture Pharmacist Astronomers Science Teacher

Importance of Science Security - The use of computer increases efficiency of a security system. Transportation - The use of vehicles reduced the time to travel from on place to another.

Communication & Information - The invention of satellite helps people interact with people around he world and - search information more quickly Medical - The production of medicine help to improve human health and cure. Agriculture - the use of machines increase the production of food.

1. 2 Science laboratory

LABORATORY It is the place where a scientist do their work. Students carry out investigations or experiments during science lesson in science laboratories.

Laboratory Rules & Safety Precaution • Never enter the lab unless a teacher is present. • Do not eat and drink or taste any chemical in the lab. • Always follow the teacher’s instruction • Never touch chemicals with your bare hands. Use a spatula.

Continue… Read the label on a reagent bottle before using its content. Turn off the Bunsen burner after use. Do not pour back any unused chemical into its bottle. Wash and keep all apparatus after use. Do not test anything without teacher’s permission. Report any breakages to the teacher.

Hazardous warning Symbols There are many substances in science lab. Some of them are hazardous. So, we use hazard warning symbols to show the danger of the substances.

Eg: Mercury, Bromine and Sodium Cyanide Effects: Causes death or harm if absorbed through skin, swallowed, or inhaled. Toxic/Poisonous Eg: Ammonia, Chloroform, Chlorine Effects: Causes discomfort and irritation to body or skin. Irritant/Harmful

Eg: Alchohol, Petrol, Kerosine Effects: Can burn very easily Flammable/Inflammable Eg: Plutonium, Uronium Effects: Gives out radiation and can cause radioactive effects Radioactive

Eg: Concentrated Acid such as Sulphuric acid Effects: Can damage the skin and eyes upon contact Corrosive Eg: Sodium, Potassium Effects: Can explode very easily Explosive

Laboratory Apparatus I will hand out the pictures of lab apparatus. Paste it in your book.

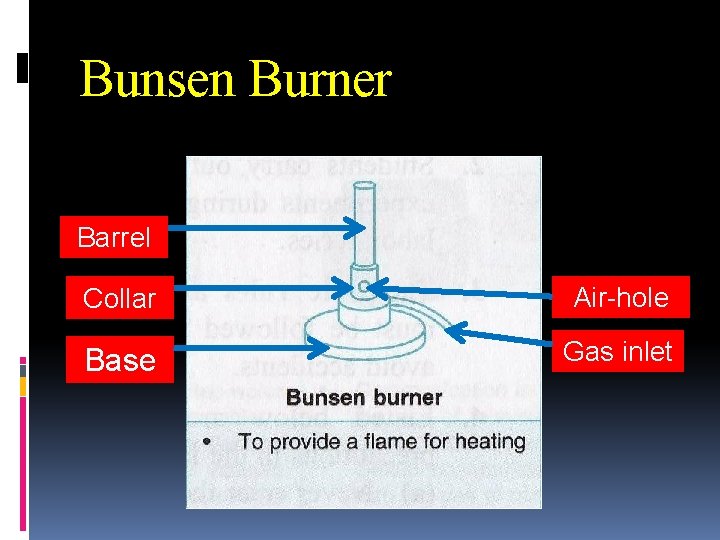
Bunsen Burner Barrel Collar Air-hole Base Gas inlet

Luminous Flame Non-luminous Flame

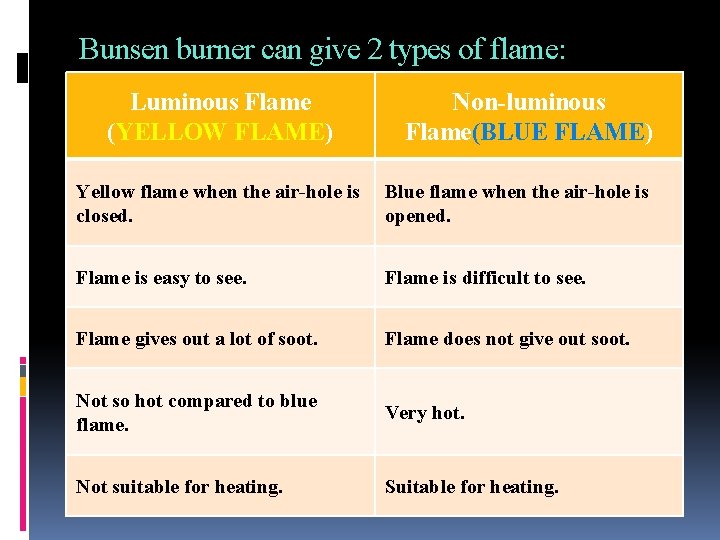
Bunsen burner can give 2 types of flame: Luminous Flame (YELLOW FLAME) Non-luminous Flame(BLUE FLAME) Yellow flame when the air-hole is closed. Blue flame when the air-hole is opened. Flame is easy to see. Flame is difficult to see. Flame gives out a lot of soot. Flame does not give out soot. Not so hot compared to blue flame. Very hot. Not suitable for heating. Suitable for heating.

How to use a Bunsen burner 1) Close the air-hole by turning the collar. 2) Hold light at the top of the barrel. 3) Turn on the gas slowly, a yellow flame will be obtained. 4) Open the air-hole to obtain a blue flame.

1. 3 Steps in scientific investigation

Process Skills in Science asking question making observation taking measurement recording the data collected analyzing and interpreting data making conclusions writing report to communicate the results

There involves 8 Steps in Scientific Investigation 1) 2) 3) 4) 5) 6) 7) 8) Identifying problems Forming a hypothesis Planning the experiment Carrying out the experiment Collecting data Analyzing the data Making a conclusion Writing a report

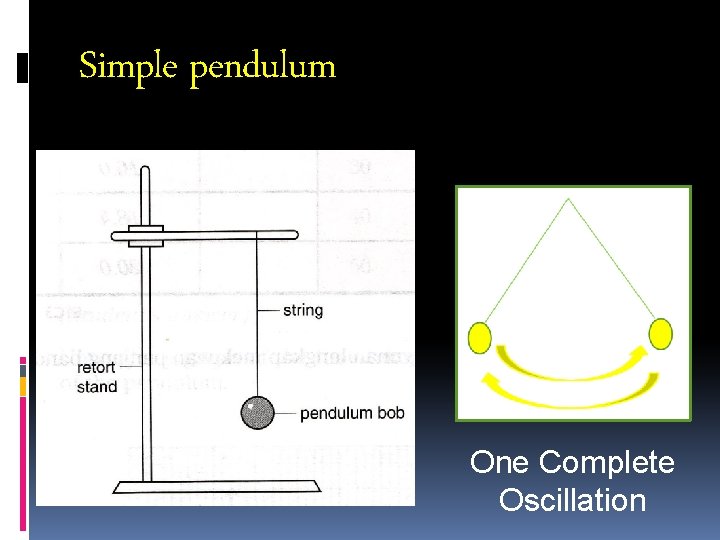
Simple pendulum One Complete Oscillation

Steps in scientific investigation: Experiment: The simple pendulum 1. Identifying problem- determining ‘what I want to find out’ usually by asking question. Eg: How does the length of a pendulum affects the period of oscillation? Forming a hypothesis- a smart guess/ possible answer to the problem Eg: The longer the length of the pendulum the longer the time taken for 10 oscillation. 2.

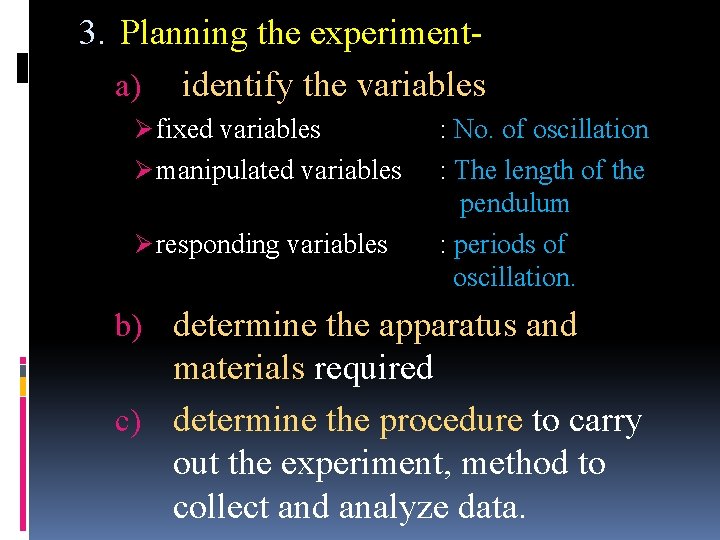
3. Planning the experiment- a) identify the variables Ø fixed variables : No. of oscillation Ø manipulated variables : The length of the pendulum Ø responding variables : periods of oscillation. b) determine the apparatus and materials required c) determine the procedure to carry out the experiment, method to collect and analyze data.

4. Carrying out the experiment- controlling the variables as planned 5. Collecting data- writing down what has been observed. In the form of sentence or table.

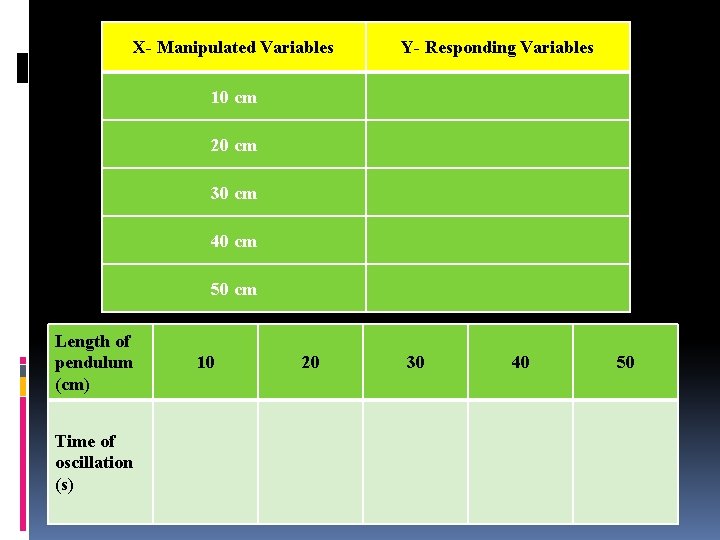
X- Manipulated Variables Y- Responding Variables 10 cm 20 cm 30 cm 40 cm 50 cm Length of pendulum (cm) Time of oscillation (s) 10 20 30 40 50

6. Analyzing the data- giving an explanation on data collected Eg: The pendulum with longer string take longer time to oscillate than the pendulum with a shorter string. 7. Making a conclusion- State whether the hypothesis is accepted or not. Eg: The longer the length of the pendulum, the longer the period of oscillation. Hypothesis is accepted.

8. Writing a report- present the … a) Aim/ Objective: To study the. . . b) Problem statement: How does…? c) Hypothesis: The … the… d) Variables: Fixed, manipulated, responding e) Apparatus: Radas f) Materials: Bahan g) Procedures/ Steps h) Result: Collected data i) Analysis j) Conclusion/ Inference: Hypothesis accepted or not

THE END