Chapter 1 Introduction to Forensic Chemistry Ch 1












- Slides: 16

Chapter 1 Introduction to Forensic Chemistry

Ch. 1 Essential Questions § What are essential questions? § Main ideas in the chapter students should focus on § What is forensic chemistry? § What are the states of matter? § What are elements & compounds? § What is the periodic table & what does it do?

What is Forensic Science? § Latin meaning in a forum § Old Rome trials took place in forum § So it means: courtroom science

First Forensics Lesson § Welcome to CSI: Worcester Academy § Fingerprinting

Physical Evidence § § § What is Matter? (3 states) Physical material of the universe Solid - what is it? Orderly arranged particles Liquid - what is it? Particles fairly far apart enough to flow § Gas - what is it? § Large distances between particles, fast moving § Matter Demo

Pure Substances § What is it? § Matter with uniform composition § Can’t be separated by physical means § 2 kinds § Elements - from Periodic Table § Compounds - 2 or more elements chemically attached

Mixtures § What is a mixture? § Combo of 2 or more pure substances § 2 kinds § Evenly distributed dissolved compound in water § Homogeneous § Composition in mixture varies § Heterogeneous § What are some examples?

The Periodic Table § What does it do? § Organizes all the elements known § Who was the 1 st guy to organize it & when? § Mendelev, 1860 s § How many elements were on the 1 st table? § 63 § He also predicted 2 elements not yet discovered - what were they? § Gallium (31) & Germanium (32

The NEW Periodic Table § § § § § Divided into 3 Main Groups: Metals Metalloids Non-metals What are some examples of the 3 different groups? Each element has own abbreviation called: Symbol, most are in what language? Latin Lets Play the Symbol Game!

Elements Make Up Compounds § What are some simple elements that combine to form compounds? § Na. Cl § H 2 O § CO 2 § CH 4 § These combos of elements are called? § Formulas

Chromatography § § § § § Separating mixtures in lab Also called color writing How it works: Add a compound to another That will attract or repel the other They separate over time TLC - what is it? Tender Love & Care - NO! Thin Layer Chromotography or mix chemicals and let them move up the paper

CSI WA: The “DEAd” Agent § What happened to Kiki Carmena? § Mexican Govt tried to frame Bravo drug family § Soil from another area found under Kiki’s nails § Mexico conspired with another drug family to kill him and kill off other drug family

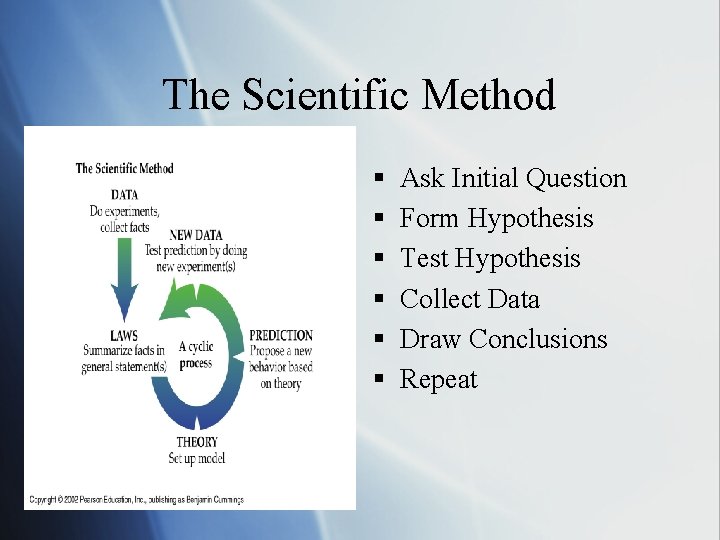
The Scientific Method § § § Ask Initial Question Form Hypothesis Test Hypothesis Collect Data Draw Conclusions Repeat

What is a Candle? Using Scientific Method § 1. State the problem § What is fuel for a candle? § 2. Form a Hypothesis § The ____ is candle’s fuel § 3. Test Hypothesis § Burn the Candle § 4. Gather Results § Write down observations of what happens § 5. Make Conclusions § Wax melted, Etc. § 6. Repeat

Different Experiment with a Candle § Take the same steps § Add a variable § Variable: Take away fuel, stop the candle § How many ways can you put out a candle?

Homework § Get 3 -Ring Binder & 5 Tabs § Read Handout (6 pgs) § Do Questions at end of Handout (5)