CHAPTER 1 Introduction to Chemical Engineering Thermodynamics Norhaniza



- Slides: 34

CHAPTER 1 Introduction to Chemical Engineering Thermodynamics Norhaniza binti Yusof Faculty of Chemical and Energy Engineering Universiti Teknologi Malaysia, 81310 UTM Johor, Johor Bahru, Malaysia Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

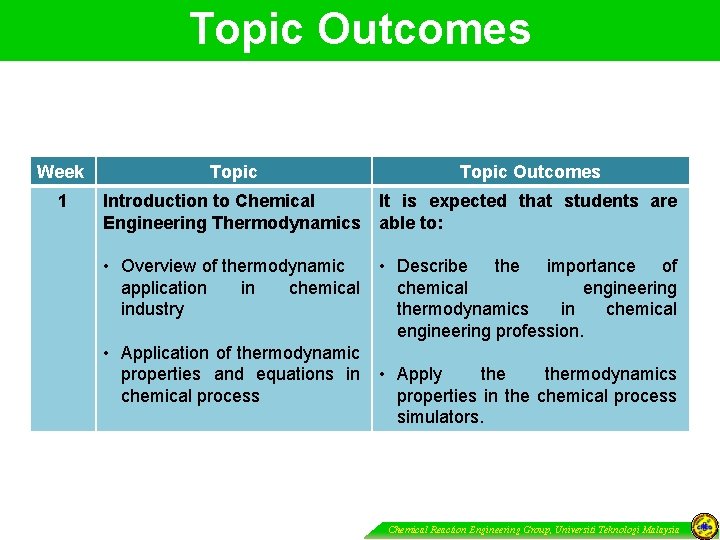
Topic Outcomes Week 1 Topic Outcomes Introduction to Chemical It is expected that students are Engineering Thermodynamics able to: • Overview of thermodynamic application in chemical industry • Application of thermodynamic properties and equations in chemical process • Describe the importance of chemical engineering thermodynamics in chemical engineering profession. • Apply thermodynamics properties in the chemical process simulators. Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

Scope of Lecture Overview of thermodynamic application in chemical industry Application of thermodynamic properties and equations in chemical process Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

Thermodynamic Applications Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

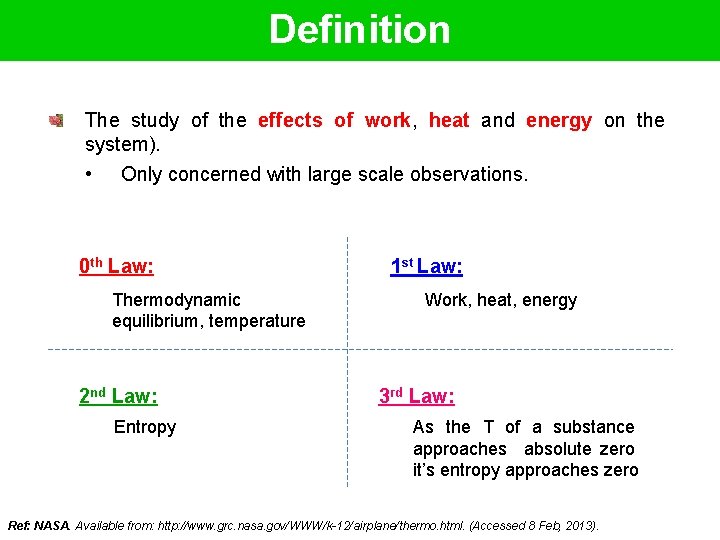
Definition The study of the effects of work, heat and energy on the system). • Only concerned with large scale observations. 0 th Law: Thermodynamic equilibrium, temperature 2 nd Law: Entropy 1 st Law: Work, heat, energy 3 rd Law: As the T of a substance approaches absolute zero it’s entropy approaches zero Ref: NASA. Available from: http: //www. grc. nasa. gov/WWW/k-12/airplane/thermo. html. (Accessed 8 Feb, 2013).

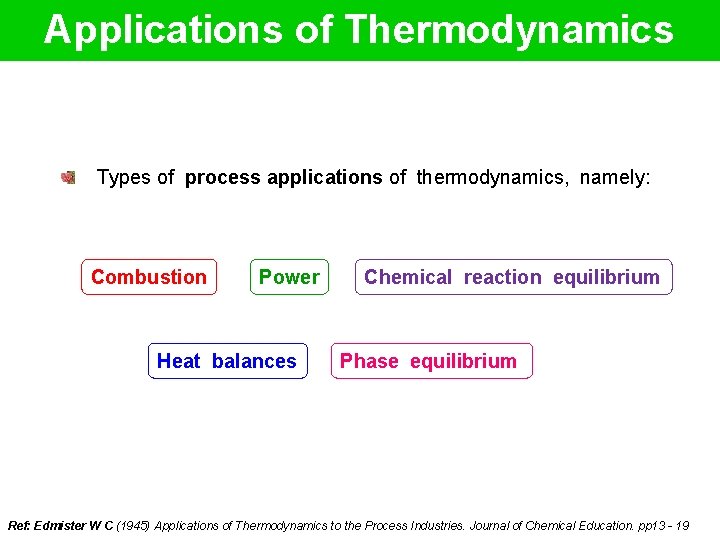
Applications of Thermodynamics Types of process applications of thermodynamics, namely: Combustion Power Heat balances Chemical reaction equilibrium Phase equilibrium Ref: Edmister W C (1945) Applications of Thermodynamics to the Process Industries. Journal of Chemical Education. pp 13 - 19

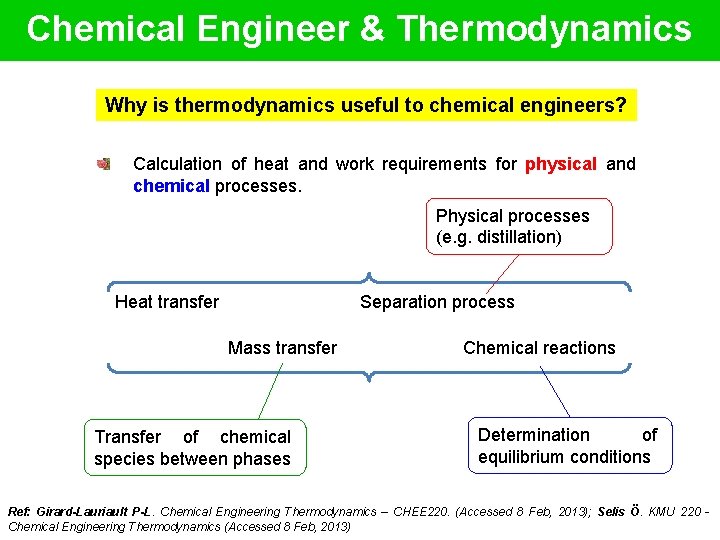
Chemical Engineer & Thermodynamics Why is thermodynamics useful to chemical engineers? Calculation of heat and work requirements for physical and chemical processes. Physical processes (e. g. distillation) Heat transfer Separation process Mass transfer Transfer of chemical species between phases Chemical reactions Determination of equilibrium conditions Ref: Girard-Lauriault P-L. Chemical Engineering Thermodynamics – CHEE 220. (Accessed 8 Feb, 2013); Selis Ö. KMU 220 Chemical Engineering Thermodynamics (Accessed 8 Feb, 2013)

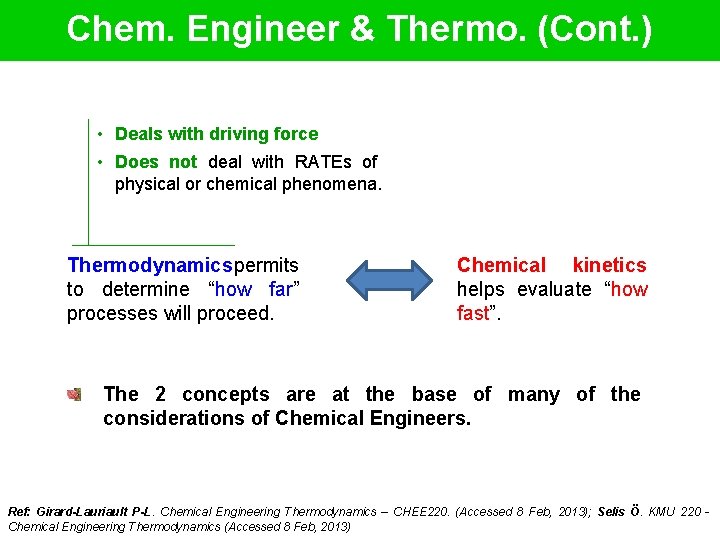
Chem. Engineer & Thermo. (Cont. ) • Deals with driving force • Does not deal with RATEs of physical or chemical phenomena. Thermodynamics permits to determine “how far” processes will proceed. Chemical kinetics helps evaluate “how fast”. The 2 concepts are at the base of many of the considerations of Chemical Engineers. Ref: Girard-Lauriault P-L. Chemical Engineering Thermodynamics – CHEE 220. (Accessed 8 Feb, 2013); Selis Ö. KMU 220 Chemical Engineering Thermodynamics (Accessed 8 Feb, 2013)

Examples Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

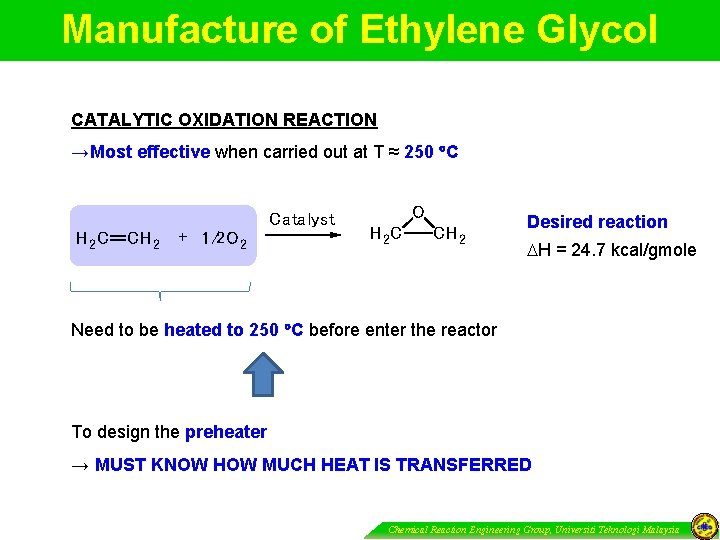
Manufacture of Ethylene Glycol CATALYTIC OXIDATION REACTION →Most effective when carried out at T ≈ 250 C Catalyst H 2 C CH 2 + 1/2 O 2 O H 2 C CH 2 Desired reaction H = 24. 7 kcal/gmole Need to be heated to 250 C before enter the reactor To design the preheater → MUST KNOW HOW MUCH HEAT IS TRANSFERRED Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

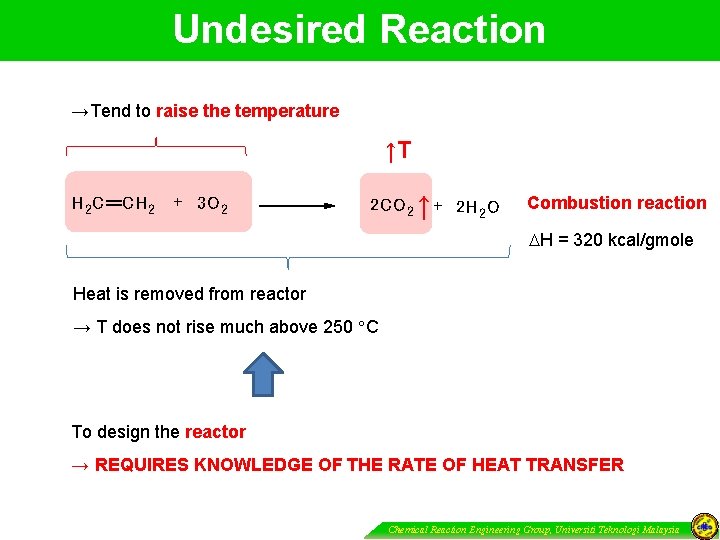
Undesired Reaction →Tend to raise the temperature ↑T H 2 C CH 2 + 3 O 2 2 CO 2 ↑ + 2 H 2 O Combustion reaction H = 320 kcal/gmole Heat is removed from reactor → T does not rise much above 250 C To design the reactor → REQUIRES KNOWLEDGE OF THE RATE OF HEAT TRANSFER Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

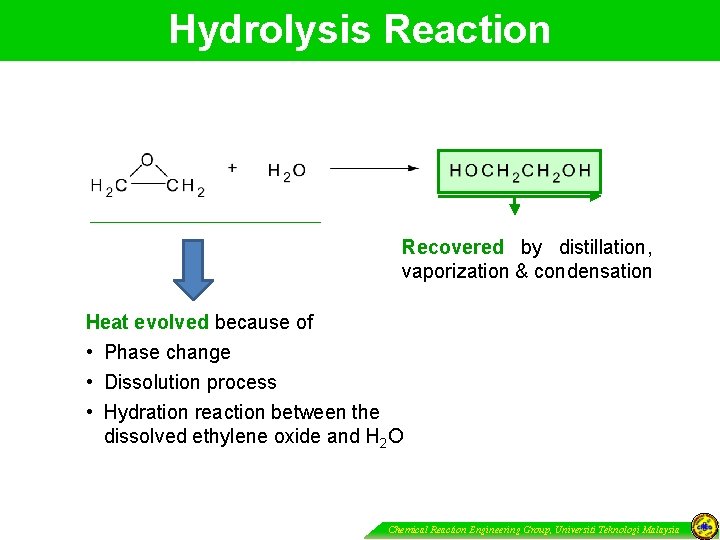
Hydrolysis Reaction Recovered by distillation, vaporization & condensation Heat evolved because of • Phase change • Dissolution process • Hydration reaction between the dissolved ethylene oxide and H 2 O Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

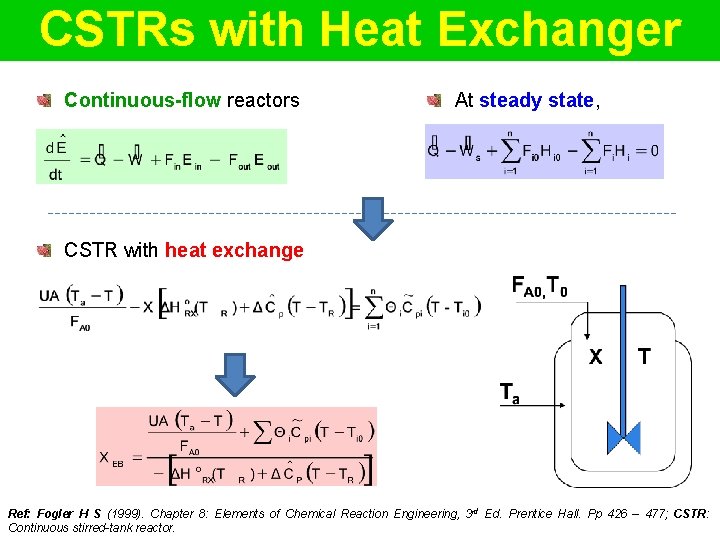
CSTRs with Heat Exchanger Continuous-flow reactors At steady state, CSTR with heat exchange Ref: Fogler H S (1999). Chapter 8: Elements of Chemical Reaction Engineering, 3 rd Ed. Prentice Hall. Pp 426 – 477; CSTR: Continuous stirred-tank reactor.

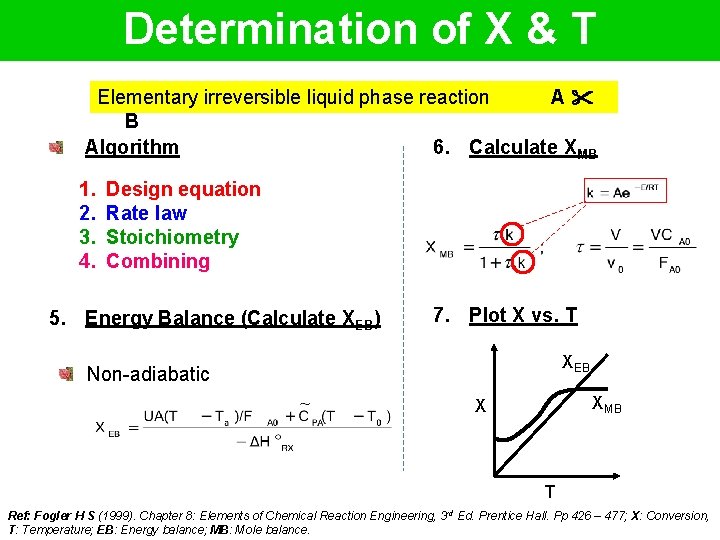
Determination of X & T Elementary irreversible liquid phase reaction A B Algorithm 6. Calculate XMB 1. 2. 3. 4. Design equation Rate law Stoichiometry Combining 5. Energy Balance (Calculate XEB) 7. Plot X vs. T XEB Non-adiabatic XMB X T Ref: Fogler H S (1999). Chapter 8: Elements of Chemical Reaction Engineering, 3 rd Ed. Prentice Hall. Pp 426 – 477; X: Conversion, T: Temperature; EB: Energy balance; MB: Mole balance.

Properties & Equations Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

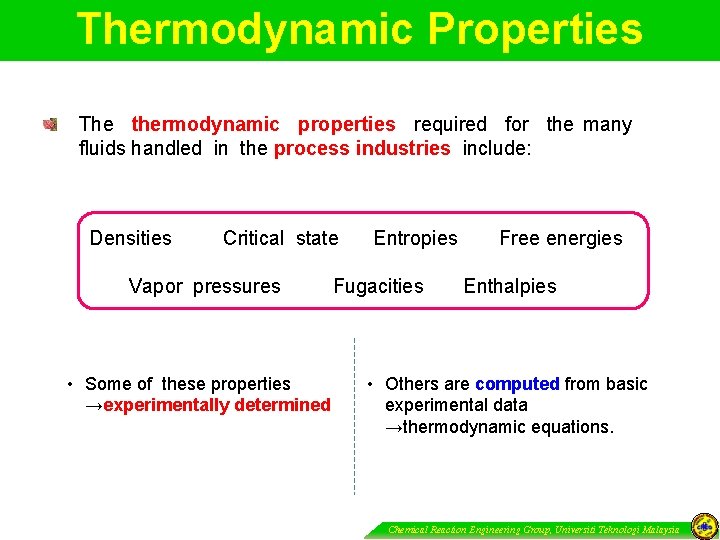
Thermodynamic Properties The thermodynamic properties required for the many fluids handled in the process industries include: Densities Critical state Vapor pressures • Some of these properties →experimentally determined Entropies Fugacities Free energies Enthalpies • Others are computed from basic experimental data →thermodynamic equations. Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

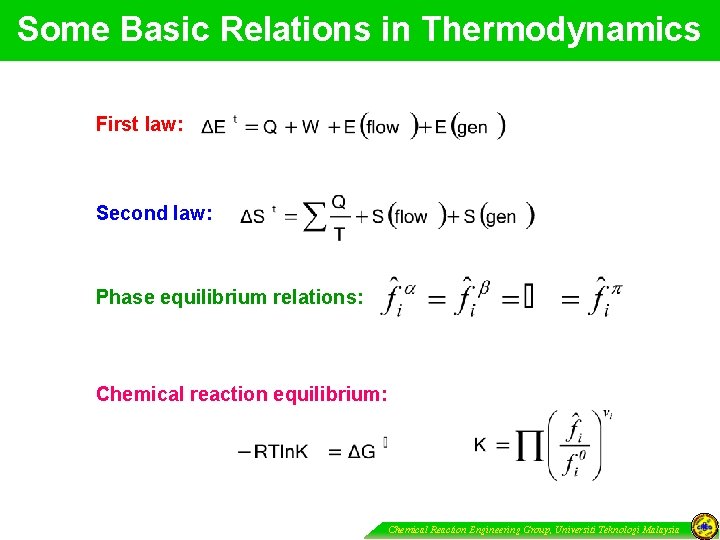
Some Basic Relations in Thermodynamics First law: Second law: Phase equilibrium relations: Chemical reaction equilibrium: Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

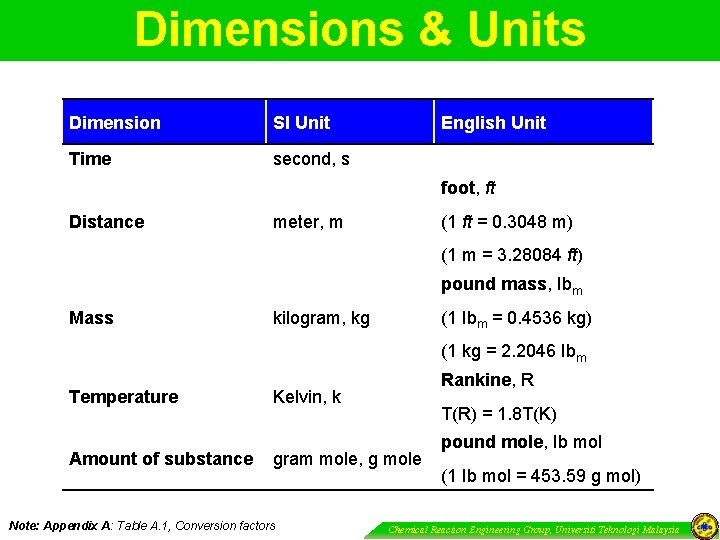
Dimensions & Units Dimension SI Unit Time second, s English Unit foot, ft Distance meter, m (1 ft = 0. 3048 m) (1 m = 3. 28084 ft) pound mass, Ibm Mass kilogram, kg (1 Ibm = 0. 4536 kg) (1 kg = 2. 2046 Ibm Temperature Amount of substance Rankine, R Kelvin, k T(R) = 1. 8 T(K) gram mole, g mole Note: Appendix A: Table A. 1, Conversion factors pound mole, Ib mol (1 Ib mol = 453. 59 g mol) Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

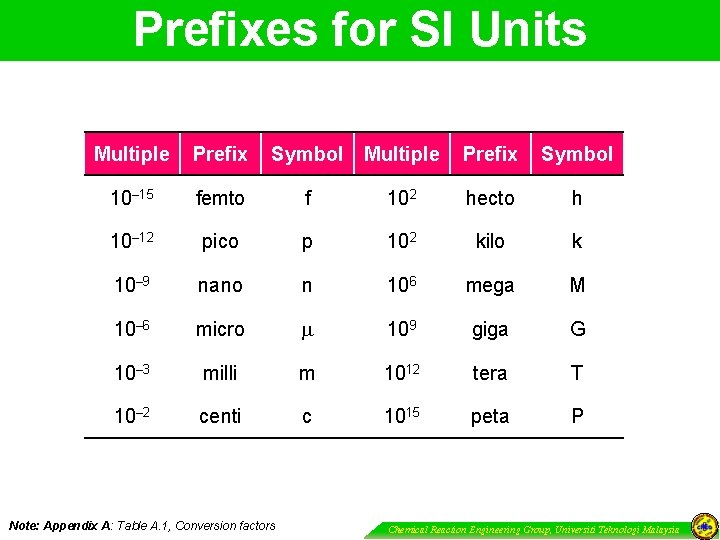
Prefixes for SI Units Multiple Prefix Symbol 10– 15 femto f 102 hecto h 10– 12 pico p 102 kilo k 10– 9 nano n 106 mega M 10– 6 micro 109 giga G 10– 3 milli m 1012 tera T 10– 2 centi c 1015 peta P Note: Appendix A: Table A. 1, Conversion factors Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

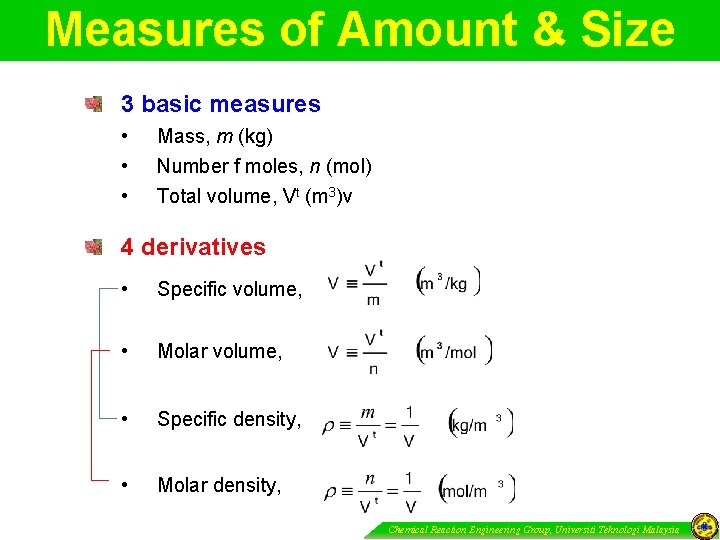
Measures of Amount & Size 3 basic measures • • • Mass, m (kg) Number f moles, n (mol) Total volume, Vt (m 3)v 4 derivatives • Specific volume, • Molar volume, • Specific density, • Molar density, Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

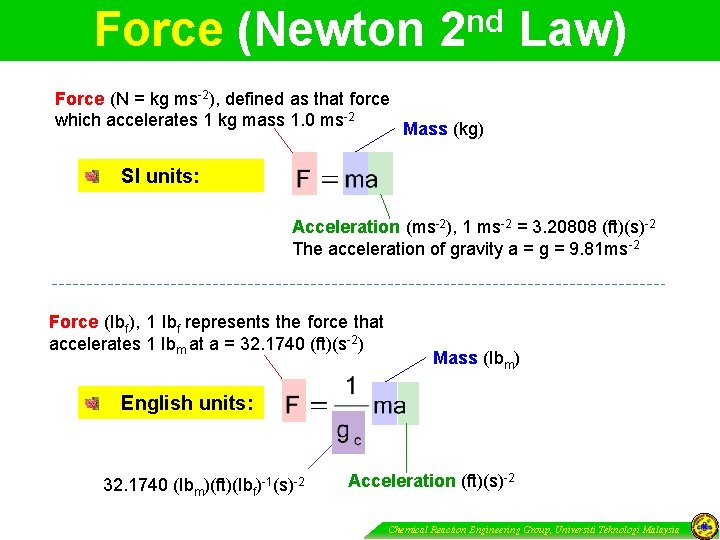
Force (Newton nd 2 Law) Force (N = kg ms-2), defined as that force which accelerates 1 kg mass 1. 0 ms-2 Mass (kg) SI units: Acceleration (ms-2), 1 ms-2 = 3. 20808 (ft)(s)-2 The acceleration of gravity a = g = 9. 81 ms-2 Force (Ibf), 1 Ibf represents the force that accelerates 1 Ibm at a = 32. 1740 (ft)(s-2) Mass (Ibm) English units: 32. 1740 (Ibm)(ft)(Ibf)-1(s)-2 Acceleration (ft)(s)-2 Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

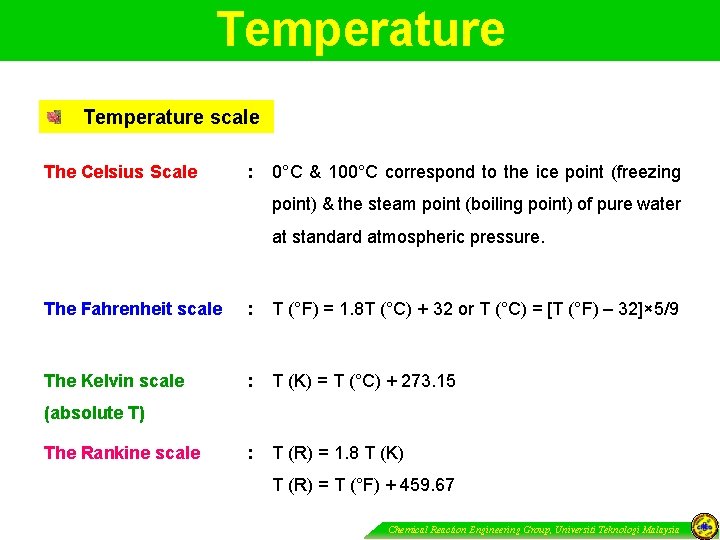
Temperature scale The Celsius Scale : 0°C & 100°C correspond to the ice point (freezing point) & the steam point (boiling point) of pure water at standard atmospheric pressure. The Fahrenheit scale : T (°F) = 1. 8 T (°C) + 32 or T (°C) = [T (°F) – 32]× 5/9 The Kelvin scale : T (K) = T (°C) + 273. 15 : T (R) = 1. 8 T (K) (absolute T) The Rankine scale T (R) = T (°F) + 459. 67 Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

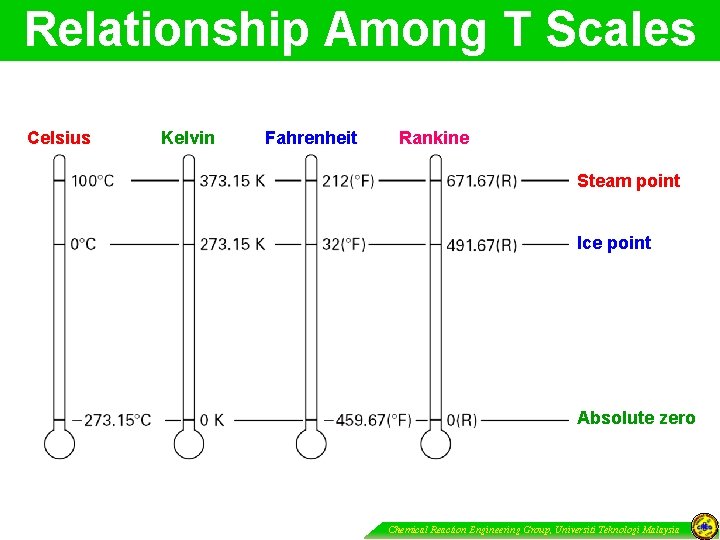
Relationship Among T Scales Celsius Kelvin Fahrenheit Rankine Steam point Ice point Absolute zero Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

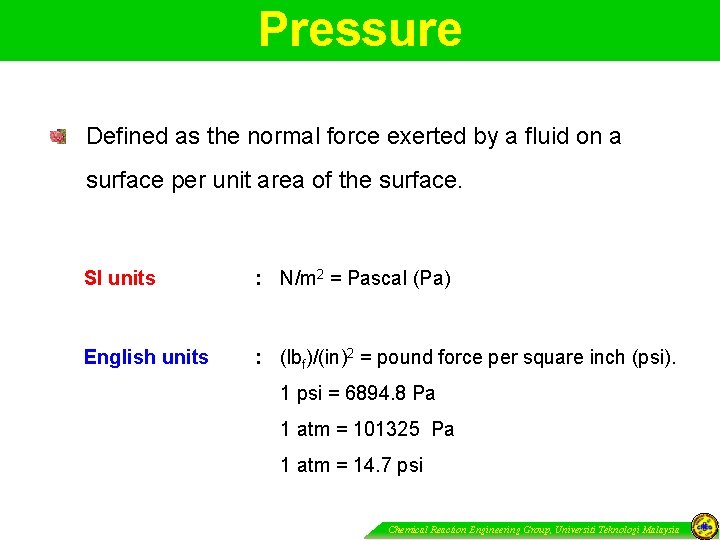
Pressure Defined as the normal force exerted by a fluid on a surface per unit area of the surface. SI units : N/m 2 = Pascal (Pa) English units : (lbf)/(in)2 = pound force per square inch (psi). 1 psi = 6894. 8 Pa 1 atm = 101325 Pa 1 atm = 14. 7 psi Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

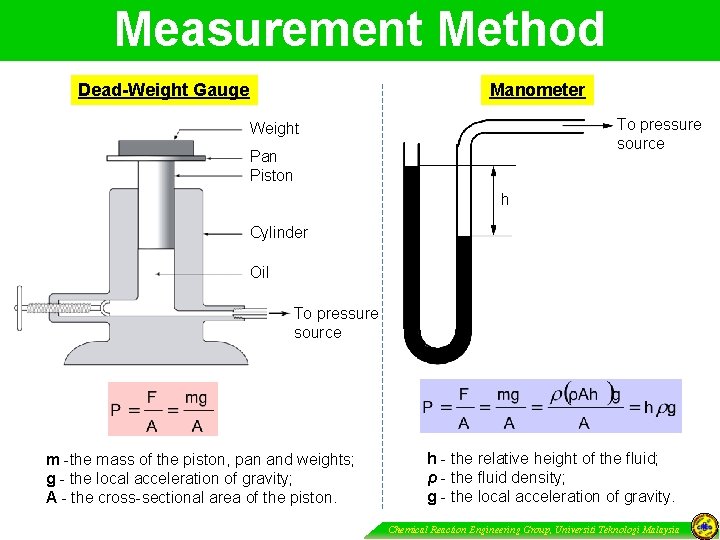
Measurement Method Manometer Dead-Weight Gauge To pressure source Weight Pan Piston h Cylinder Oil To pressure source m -the mass of the piston, pan and weights; g - the local acceleration of gravity; A - the cross-sectional area of the piston. h - the relative height of the fluid; ρ - the fluid density; g - the local acceleration of gravity. Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

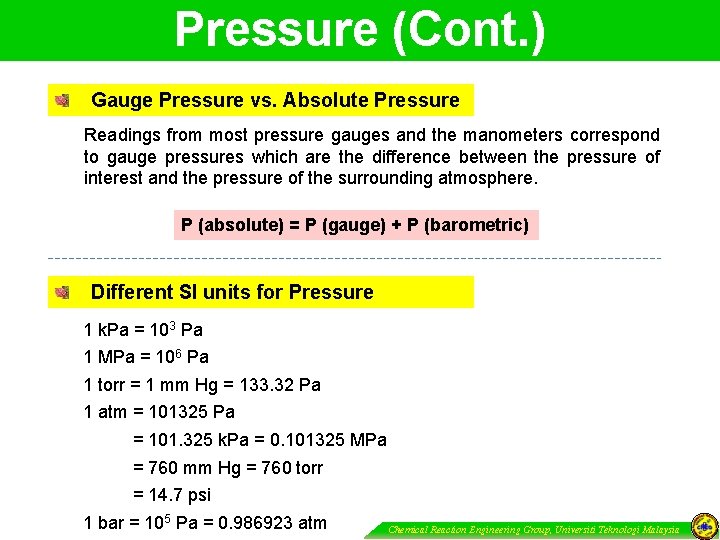
Pressure (Cont. ) Gauge Pressure vs. Absolute Pressure Readings from most pressure gauges and the manometers correspond to gauge pressures which are the difference between the pressure of interest and the pressure of the surrounding atmosphere. P (absolute) = P (gauge) + P (barometric) Different SI units for Pressure 1 k. Pa = 103 Pa 1 MPa = 106 Pa 1 torr = 1 mm Hg = 133. 32 Pa 1 atm = 101325 Pa = 101. 325 k. Pa = 0. 101325 MPa = 760 mm Hg = 760 torr = 14. 7 psi 1 bar = 105 Pa = 0. 986923 atm Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

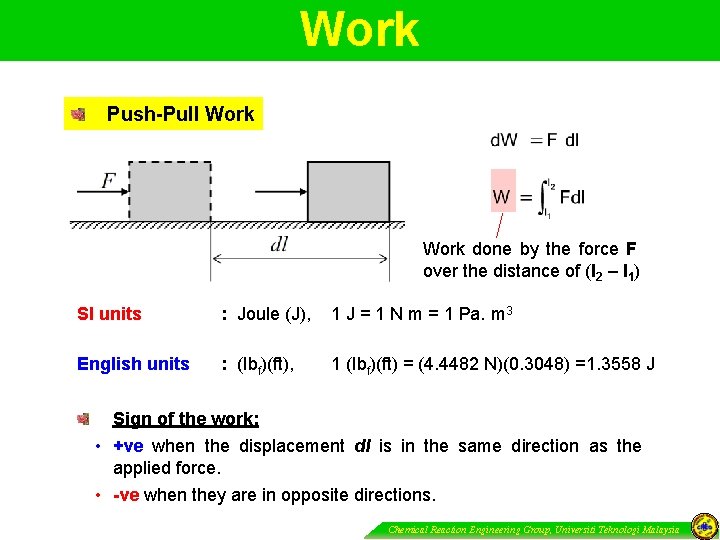
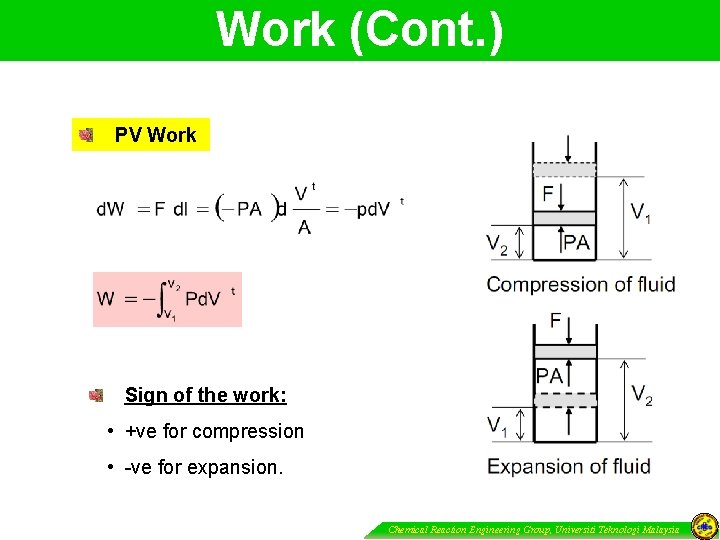
Work Push-Pull Work done by the force F over the distance of (l 2 – l 1) SI units : Joule (J), 1 J = 1 N m = 1 Pa. m 3 English units : (Ibf)(ft), 1 (Ibf)(ft) = (4. 4482 N)(0. 3048) =1. 3558 J Sign of the work: • +ve when the displacement dl is in the same direction as the applied force. • -ve when they are in opposite directions. Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

Work (Cont. ) PV Work Sign of the work: • +ve for compression • -ve for expansion. Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

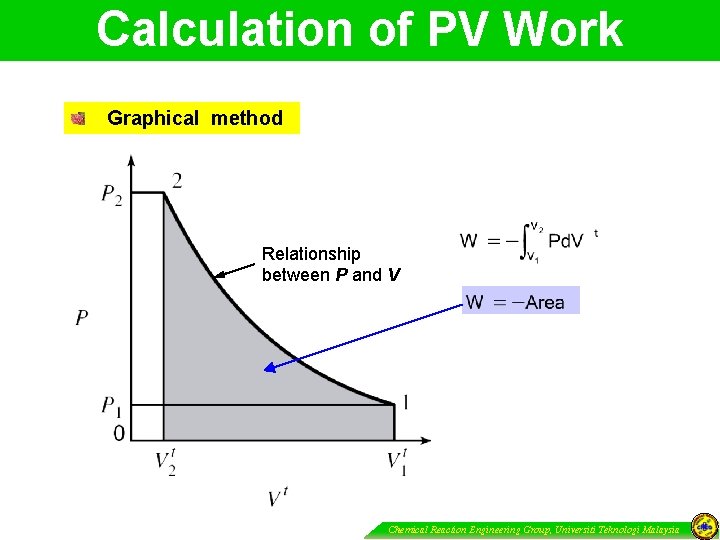
Calculation of PV Work Graphical method Relationship between P and V Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

Energy is something that a body can store, and which it can receive or give away as work or heat. Thus, energy, work and heat are closely related. Work and heat are “energy in transit”, and are never regarded as residing in a body. Energy, work and heat have the same units: Joule (SI) or lb ft (English) Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

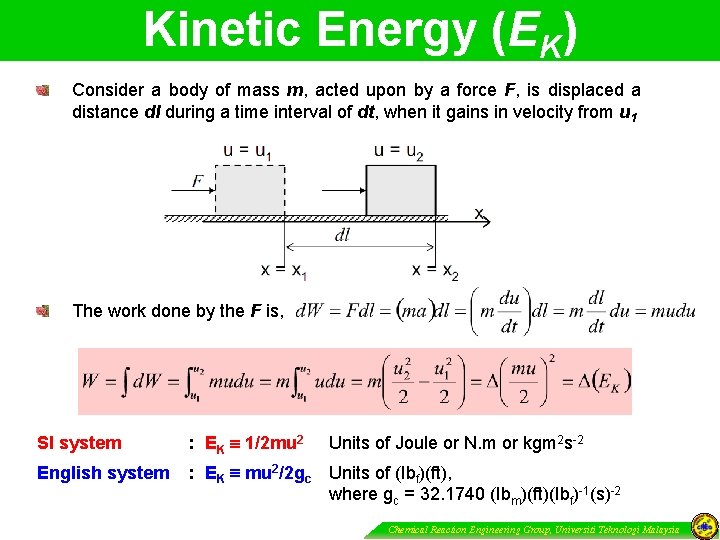
Kinetic Energy (EK) Consider a body of mass m, acted upon by a force F, is displaced a distance dl during a time interval of dt, when it gains in velocity from u 1 The work done by the F is, SI system : EK 1/2 mu 2 English system : EK mu 2/2 gc Units of (Ibf)(ft), where gc = 32. 1740 (Ibm)(ft)(Ibf)-1(s)-2 Units of Joule or N. m or kgm 2 s-2 Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

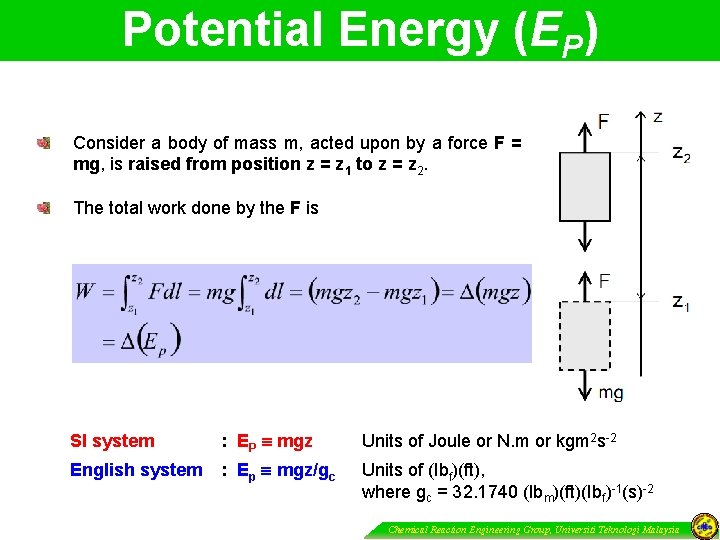
Potential Energy (EP) Consider a body of mass m, acted upon by a force F = mg, is raised from position z = z 1 to z = z 2. The total work done by the F is SI system : EP mgz Units of Joule or N. m or kgm 2 s-2 English system : Ep mgz/gc Units of (Ibf)(ft), where gc = 32. 1740 (Ibm)(ft)(Ibf)-1(s)-2 Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

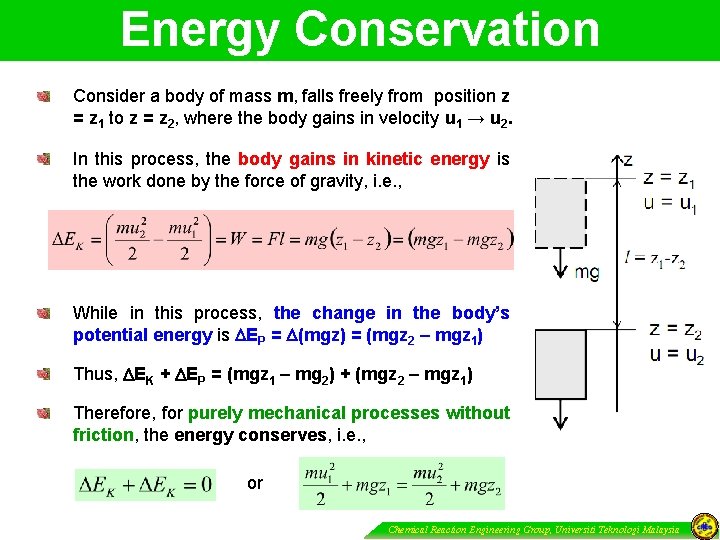
Energy Conservation Consider a body of mass m, falls freely from position z = z 1 to z = z 2, where the body gains in velocity u 1 → u 2. In this process, the body gains in kinetic energy is the work done by the force of gravity, i. e. , While in this process, the change in the body’s potential energy is EP = (mgz) = (mgz 2 – mgz 1) Thus, EK + EP = (mgz 1 – mg 2) + (mgz 2 – mgz 1) Therefore, for purely mechanical processes without friction, the energy conserves, i. e. , or Chemical Reaction Engineering Group, Universiti Teknologi Malaysia

Heat (Q) always transfers from a high temp. body to a lower temp. one. The rate of heat transfer ( ) is proportional to the temp. difference ΔT Like work, heat exists only as “energy in transit” from one body to another or between a system and its surroundings. When energy in the form of heat is added to a system, this part of energy is stored NOT as heat, but as kinetic and potential energy of atoms/molecules in the system. Units of heat SI system : Joule (J) Calorie (Cal), British system 1 Cal = 4. 184 J : (Ibf)(ft), 1 (Ibf)(ft) = 1. 3558 J British thermal Unit (Btu), 1 (Btu) = 1055. 04 J Chemical Reaction Engineering Group, Universiti Teknologi Malaysia