Chapter 1 Introduction to Biomanufacturing Northeast Biomanufacturing Center

Chapter 1 - Introduction to Biomanufacturing Northeast Biomanufacturing Center and Collaborative

What is Biomanufacturing ? § Advanced-technology manufacturing industry within biotechnology that is responsible for making biopharmaceuticals (biologics) and other bioproducts such as biofuels and human tissues. § A biopharmaceutical/biologic is any biotechnology-based therapeutic that structurally mimics components found in a living organism.

Examples of Biopharmaceuticals • • Hormones (Insulin) growth factors blood proteins clotting factors (t. PA) enzymes antibodies DNA and RNA stem cells

Applications of Biopharmaceuticals in Health & Medicine • Therapeutic proteins for treatment of disease • Vaccines to prevent disease • Protein or DNA-based diagnostics • Regenerative medicine technologies • Gene therapy

Approved Biologics Biopharmaceutical Date of Commercial Production, Indication Company Humulin (human insulin) 1982, diabetes Eli Lilly t. PA (tissue plasminogen activator 1987, myocardial infarction Genetech Humatrope (human growth hormone) 1987, human growth hormone deficiencies Eli Lilly Epogen 1989, anemia Amgen Energix-B 1989, hepatitis B vaccine Smith. Kline Beecham Betaseron 1989, multiple sclerosis Berlex Laboratories/ Chiron Ceredase 1991, Type I Gaucher’s disease Genzyme Proleukin (Il-2) 1992, kidney carcinoma Chiron AHF (recombinant anti-hemolytic factor) 1992, hemophilia A Baxter Healthcare Pulmozyme (DNAase) 1993, cystic fibrosis Genentech

Biopharmaceutical Drug Development Objective § To develop a robust, scalable, reproducible, and cost-effective process that results in safe and efficacious biopharmaceuticals § Time consuming and expensive! • 8 -15 years • $500 million to $1 billion • 1/10, 000 drug candidates makes it

Drug Development Process

Discovery/ Research § Identify gene/protein of interest § Create an “expression vector” that includes the gene of interest § Select cell type to transform § Cellular machinery transcribes DNA into m. RNA and m. RNA into protein via the Central Dogma
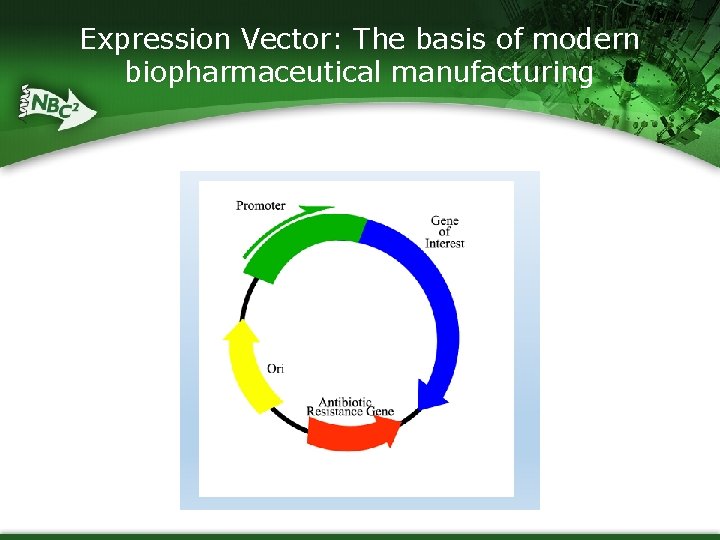
Expression Vector: The basis of modern biopharmaceutical manufacturing

Bacterial Transformation in Biomanufacturing
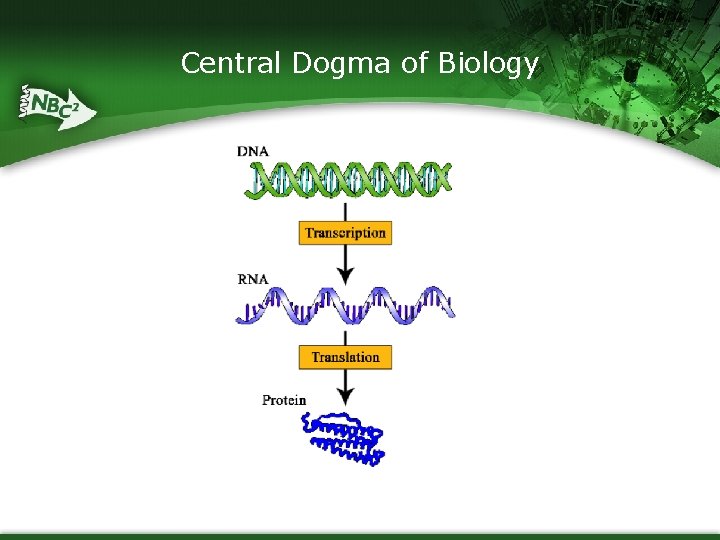
Central Dogma of Biology

Expression Systems- Considerations Post-translational modification – Modification of an amino acid of the polypeptide chain – Addition of sugar moieties to certain amino acid side chains(glycosylation) – Approximately 70% of human proteins are glycosylated and biological activity is highly affected without this modification – Majority of prokaryotic cells lack the ability to make glycoproteins

Prokaryotic Expression Systems Bacterial Systems (e. g E. Coli) • Less time consuming/expensive to culture • Unable to perform post-translational glycosylation Yeast Cultures • Less time consuming/ Inexpensive • Can add non-human glycans which are immunogenic
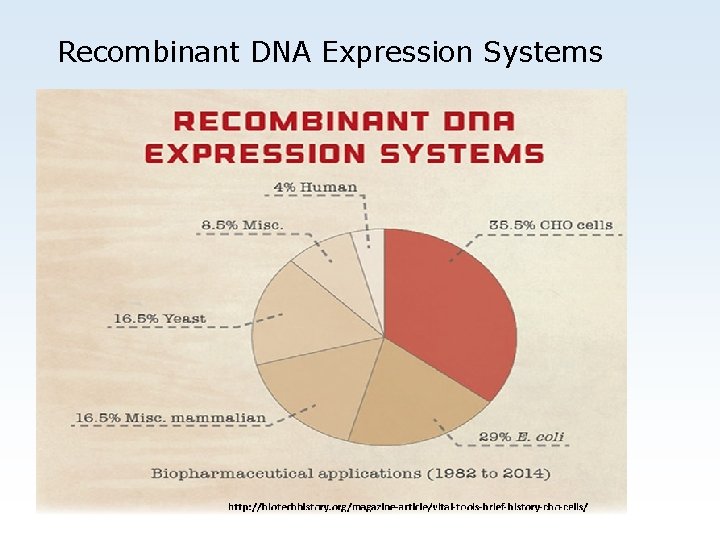
Recombinant DNA Expression Systems Expression System Application (%) CHO 35. 5 E. Coli 29 Miscellaneous mammalian 16. 5 Yeast 16. 5 Miscellaneous 8. 5 Human 4
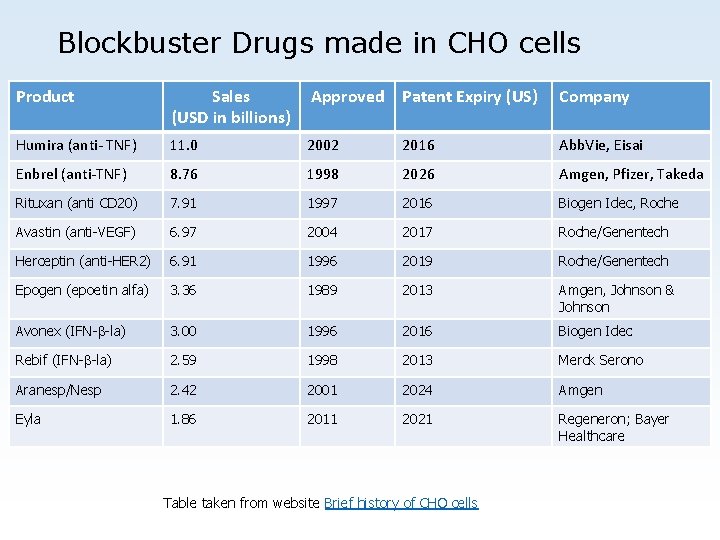
Blockbuster Drugs made in CHO cells Product Sales (USD in billions) Approved Patent Expiry (US) Company Humira (anti- TNF) 11. 0 2002 2016 Abb. Vie, Eisai Enbrel (anti-TNF) 8. 76 1998 2026 Amgen, Pfizer, Takeda Rituxan (anti CD 20) 7. 91 1997 2016 Biogen Idec, Roche Avastin (anti-VEGF) 6. 97 2004 2017 Roche/Genentech Herceptin (anti-HER 2) 6. 91 1996 2019 Roche/Genentech Epogen (epoetin alfa) 3. 36 1989 2013 Amgen, Johnson & Johnson Avonex (IFN-b-la) 3. 00 1996 2016 Biogen Idec Rebif (IFN-b-la) 2. 59 1998 2013 Merck Serono Aranesp/Nesp 2. 42 2001 2024 Amgen Eyla 1. 86 2011 2021 Regeneron; Bayer Healthcare Table taken from website Brief history of CHO cells

Eukaryotic Expression Systems Mammalian Systems – Chinese Hamster Ovary Cells (CHO) § Have the ability to perform post-translational glycosylation § Widely used but expensive and more time consuming to culture § Protein products are easier to process later in production because they are secreted into culture media
Process Development and Scale-Up Determine best processes to produce the most protein/drug as efficiently as possible. • Upstream Processing- develop equipment and processes used in production to culture the cells that make the biopharmaceutical • Downstream Processing –recovery & purification of drug from cell culture media • Formulation/Distribution –product is filled, tested for purity and sterility and distributed for clinical use

Upstream Processing § Develop equipment and processes used in production to culture the cells which produces the biopharmaceutical • Culture Flasks • Spinner Flasks • Bioreactors
Downstream Processing Recovery and purification of drug product from cell culture • Centrifugation- Ultracentrifuges • Filtration-TFF Units • Chromatography- FPLC/HPLC

Testing Finished Product § The biopharmaceutical is placed in solution, with excipients, for parenteral injection. Product tested for purity, stability, and sterility before distribution • Purity Tests • Chromatography • Electrophoresis • ELISA • Sterility Tests • Microbial Tests for endotoxins

Regulation of Biopharmaceutical Manufacturing • The manufacture of all pharmaceutical products are regulated by the Food & Drug Administration (FDA) to ensure quality and safety. • FDA requires that all pharmaceuticals be manufactured and quality of drug products using Current Good Manufacturing Processes or c. GMP

Ensuring Quality Drug Manufacturing Facilities have departments/groups that ensure quality: – Quality assurance (QA) Dept ensures that the manufacturing facility complies to all regulations and maintains all documentation for compliance – Quality Control (QC) Dept is responsible for testing the raw materials and the product during many stages of the manufacturing process

Documentation in Pharmaceutical Manufacturing Documentation can take many forms: § Process and production document (SOPs, batch records, manufacturing operations) § Operational and equipment log books (documentation of maintenance, cleaning) § Training documents § Electronic Documents
Biopharmaceutical Biomanufacturing • The production of a new biopharmaceutical may require a facility to be prepared for its manufacture and 400 to 600 people hired; 50% may be technicians • The largest bioreactor in such a facility could be 20, 000 L (scaled-up is from a 1 -2 m. L cryovial taken from the working cell bank) • Once constructed and commissioned, the facility’s equipment and process SOPs must undergo validation • The set up, maintenance and use of each piece of equipment is logged; the equipment must be calibrated • Environmental Health and Safety (EH&S) requirements are of central importance.

Amgen-Biomanufacturing Facility Tour Visit Amgen website for a virtual tour of their manufacturing facility http: //www. amgenbiotech. com/tour/amgen-manufacturing. html

Top of 20, 000 L Bioreactor
- Slides: 26