Chapter 1 Introduction 1 1 Introduction safe durable

Chapter 1. Introduction § 1. 1 Introduction • 기계, 구조물은 주어진 역할뿐 아니라 safe & durable • excess deformation, cracking must be avoided study of : mechanical behavior of materials physical testing of materials properties 필요 • design to avoid structual failure stress < strength of materials └> for deform or cracking failure 1. Stresses are often present that act in more than one direction, that is, the state of stress is biaxial or triaxial. 2. Real components may contain flaws or even cracks that must be specifically considered. 3. Stresses may be applied for long periods of time. 4. Stresses may be repeatedly applied and removed, or the direction of stress repeatedly reversed.
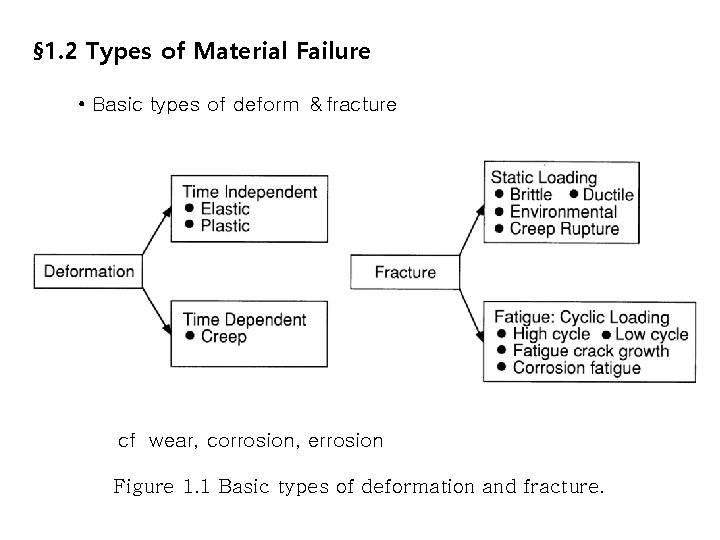
§ 1. 2 Types of Material Failure • Basic types of deform &fracture cf wear, corrosion, errosion Figure 1. 1 Basic types of deformation and fracture.
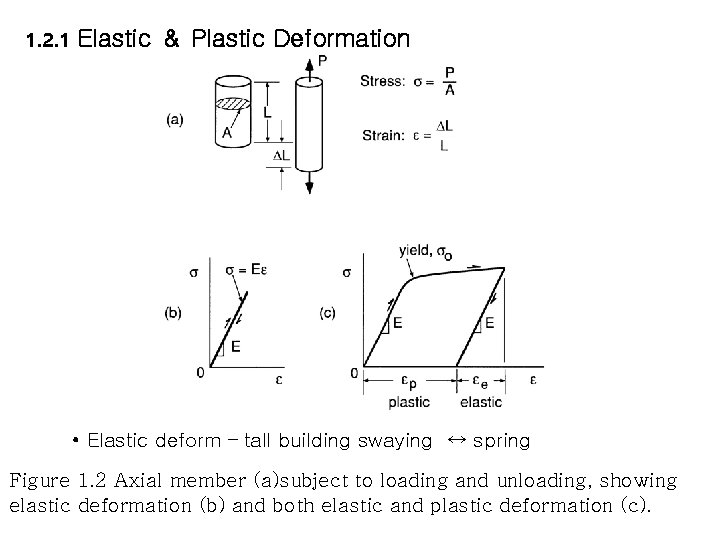
1. 2. 1 Elastic & Plastic Deformation • Elastic deform – tall building swaying ↔ spring Figure 1. 2 Axial member (a)subject to loading and unloading, showing elastic deformation (b) and both elastic and plastic deformation (c).
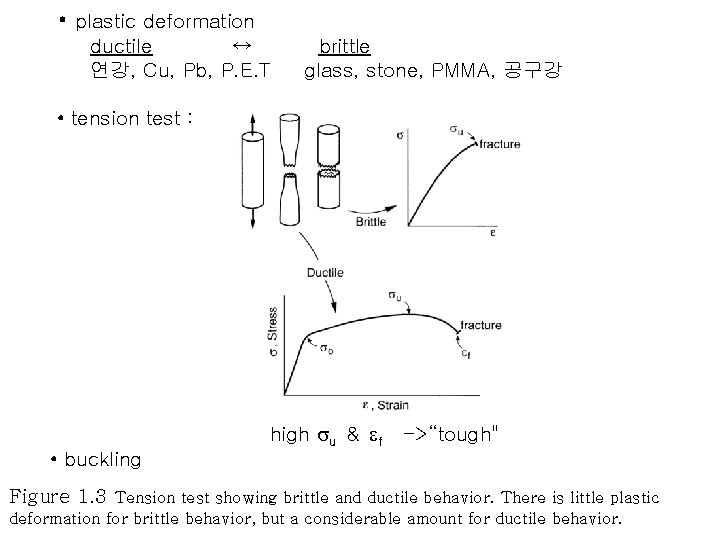
• plastic deformation ductile ↔ 연강, Cu, Pb, P. E. T brittle glass, stone, PMMA, 공구강 • tension test : high su & ef ->“tough" • buckling Figure 1. 3 Tension test showing brittle and ductile behavior. There is little plastic deformation for brittle behavior, but a considerable amount for ductile behavior.
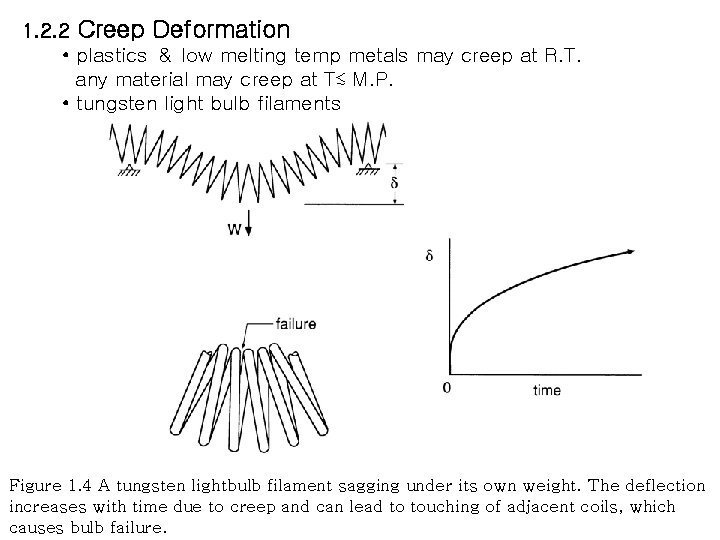
1. 2. 2 Creep Deformation • plastics & low melting temp metals may creep at R. T. any material may creep at T≲ M. P. • tungsten light bulb filaments Figure 1. 4 A tungsten lightbulb filament sagging under its own weight. The deflection increases with time due to creep and can lead to touching of adjacent coils, which causes bulb failure.
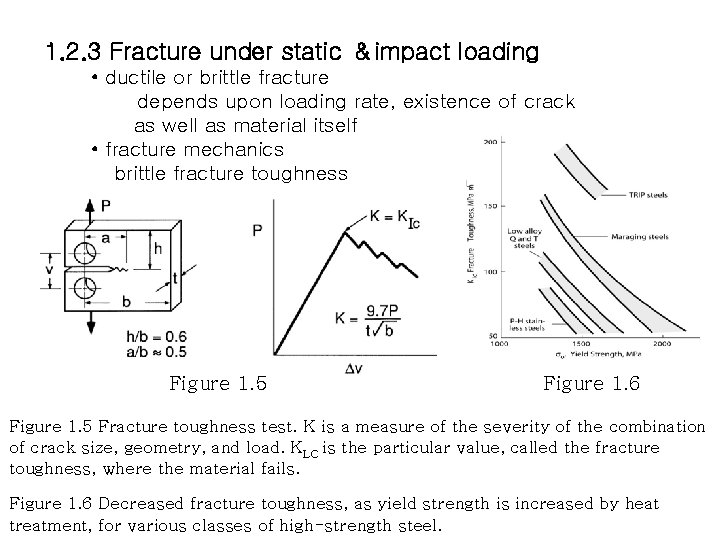
1. 2. 3 Fracture under static &impact loading • ductile or brittle fracture depends upon loading rate, existence of crack as well as material itself • fracture mechanics brittle fracture toughness Figure 1. 5 Figure 1. 6 Figure 1. 5 Fracture toughness test. K is a measure of the severity of the combination of crack size, geometry, and load. KLC is the particular value, called the fracture toughness, where the material fails. Figure 1. 6 Decreased fracture toughness, as yield strength is increased by heat treatment, for various classes of high-strength steel.

• environmental cracking ex) stress corrosion cracking Figure 1. 7 Stainless steel wires broken as a result of environmental attack. These were employed in a filter exposed at 300 o. C to a complex organic environment that included molten nylon. Cracking occurred along the boundaries of the crystal grains of the material.

1. 2. 4 Fatigue Under Cyclic Loading • crack growth under repeated loading→ failure Figure 1. 8 Development of a fatigue crack during rotating bending of a precipitationhardened aluminum alloy. Photographs at various numbers of cycles are shown for a tes requiring 400, 000 cycles for failure. The sequence in the bottom row of photographs shows more detail of the middle portion of the sequence in the top row.

ex) Figure 1. 9 Fatigue failure of a garage door spring that occurred after 15 years of service.

ex) Figure 1. 10 Main mast region of a helicopter, showing inboard ends of blades, their attachment, and the linkages and mechanism that control the pitch angles of the rotating blades. The cylinder above the rotors is not ordinarily present, but is part of instrumentation used to monitor strains in the rotor blades for experimental purposes.
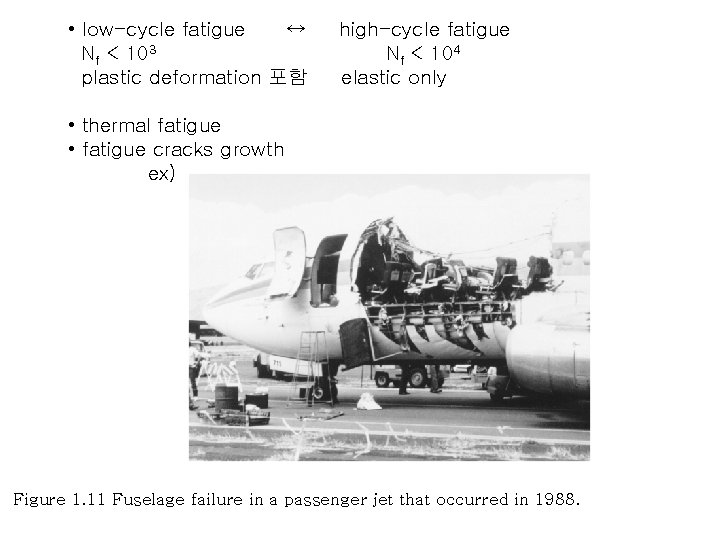
• low-cycle fatigue ↔ Nf < 103 plastic deformation 포함 high-cycle fatigue Nf < 104 elastic only • thermal fatigue • fatigue cracks growth ex) Figure 1. 11 Fuselage failure in a passenger jet that occurred in 1988.
1. 2. 5 combined effects ・ synergistic effect ex) creep + fatigue - gas or steam turbine ex) wear + fatigue - fretting fatigue ex) corrosion + fatigue - corrosion fatigue ・ environmental effect ex) UV on plastic wood in moisture, neutron radiation on steel § 1. 3 design & material selection ・ design ~ to assure the intended performance also additional requirement p. 11 설명 ・ safety & durability
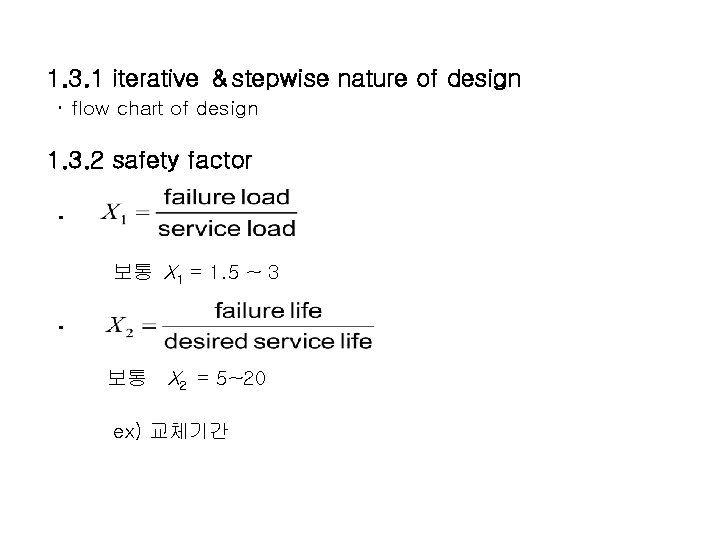
1. 3. 1 iterative &stepwise nature of design ・ flow chart of design 1. 3. 2 safety factor ・ 보통 X 1 = 1. 5 ~ 3 ・ 보통 X 2 = 5~20 ex) 교체기간

1. 3. 3 prototype &component testing ・ prototype ~ simulated service test ex) large item ex) airplane – 부분별로 component testing • computer aided simulation Figure 1. 13 Road simulation test of an automobile, with loads applied at all four wheels and the bumper mount.
1. 3. 4 Service Experience → feedback to design ex) recalls of automobile § 1. 4 Technological challenge ・ history
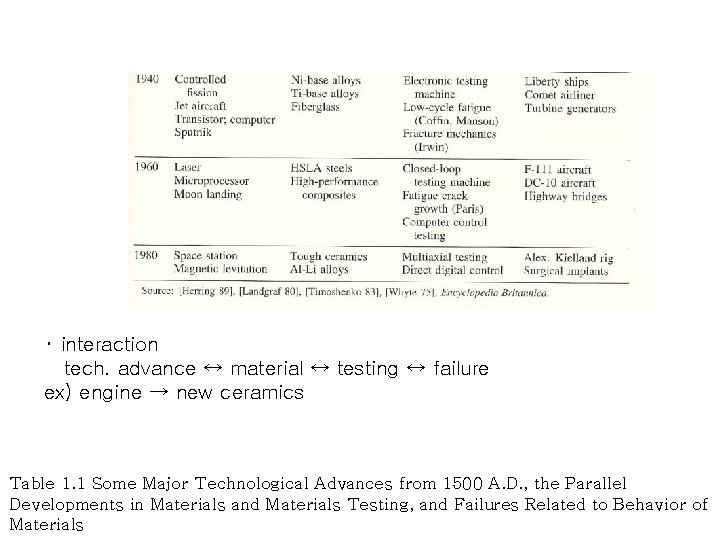
・ interaction tech. advance ↔ material ↔ testing ↔ failure ex) engine → new ceramics Table 1. 1 Some Major Technological Advances from 1500 A. D. , the Parallel Developments in Materials and Materials Testing, and Failures Related to Behavior of Materials
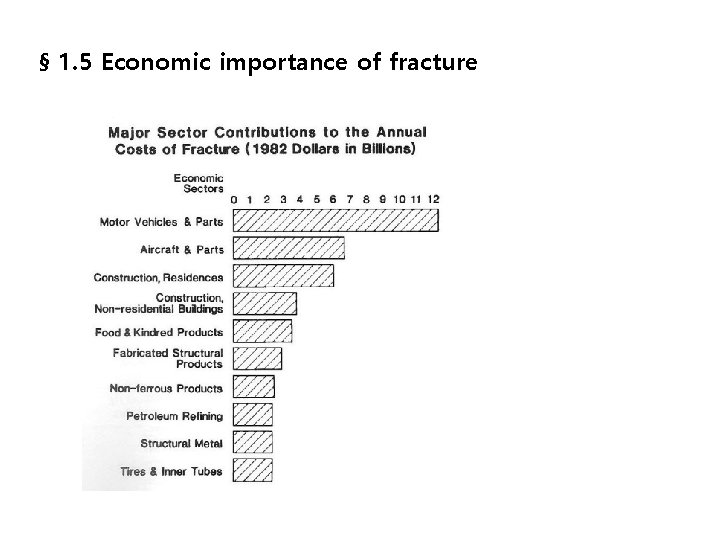
§ 1. 5 Economic importance of fracture
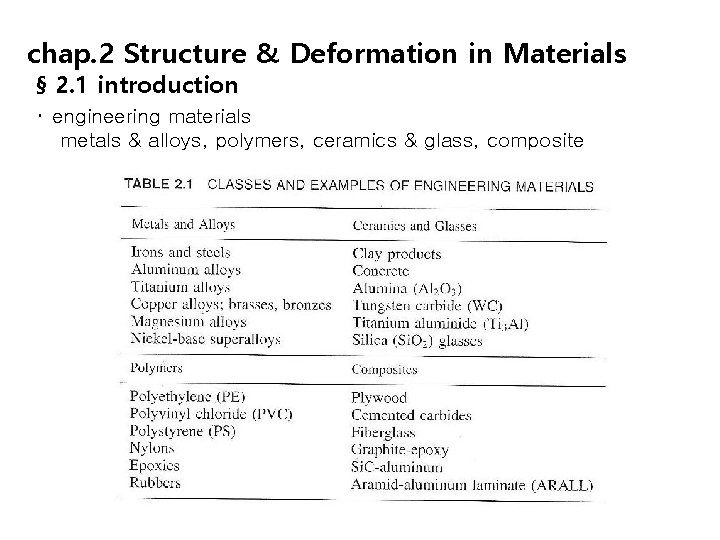
chap. 2 Structure & Deformation in Materials § 2. 1 introduction ・ engineering materials metals & alloys, polymers, ceramics & glass, composite
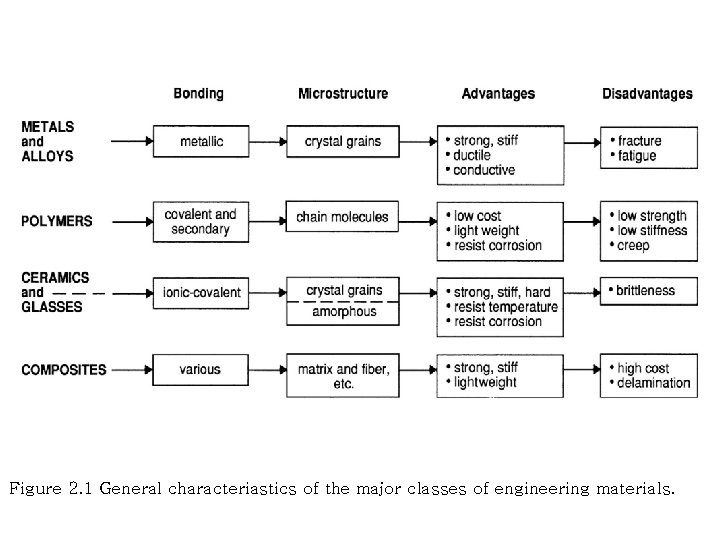
Figure 2. 1 General characteriastics of the major classes of engineering materials.
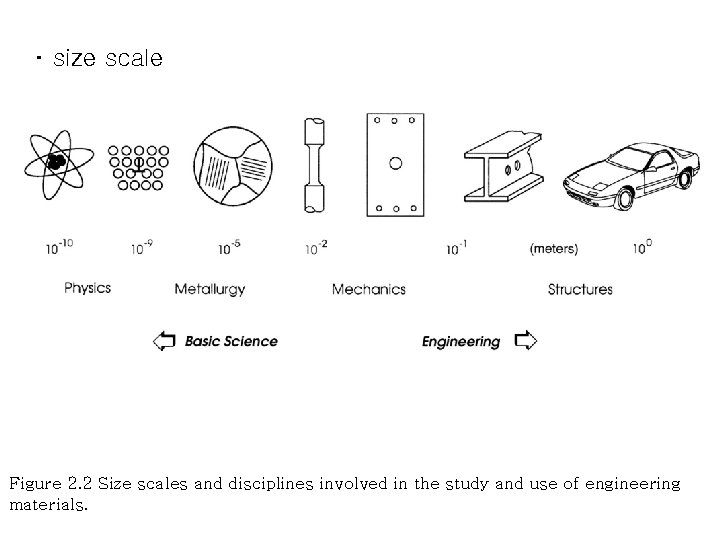
・ size scale Figure 2. 2 Size scales and disciplines involved in the study and use of engineering materials.
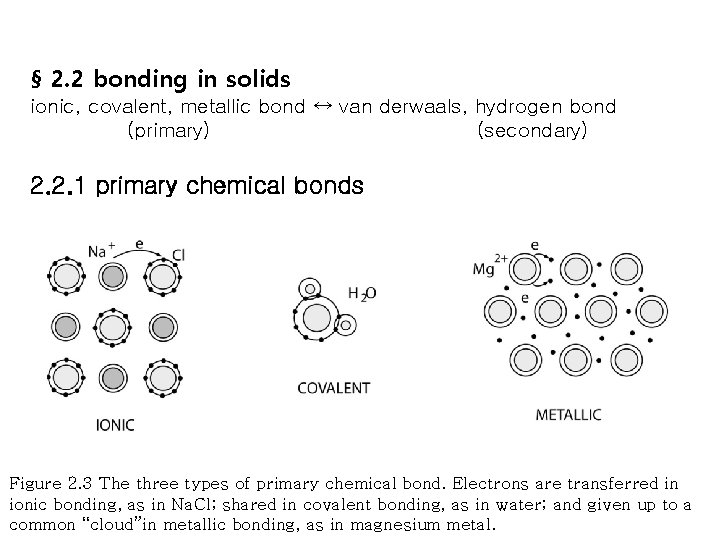
§ 2. 2 bonding in solids ionic, covalent, metallic bond ↔ van derwaals, hydrogen bond (primary) (secondary) 2. 2. 1 primary chemical bonds Figure 2. 3 The three types of primary chemical bond. Electrons are transferred in ionic bonding, as in Na. Cl; shared in covalent bonding, as in water; and given up to a common “cloud”in metallic bonding, as in magnesium metal.
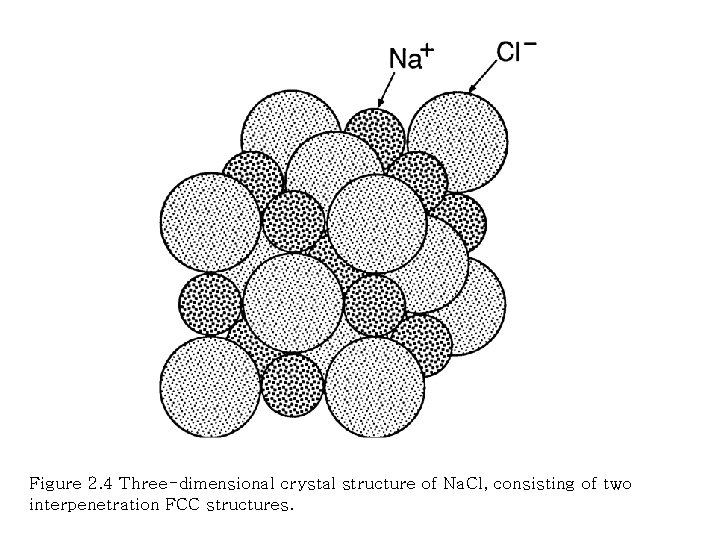
Figure 2. 4 Three-dimensional crystal structure of Na. Cl, consisting of two interpenetration FCC structures.
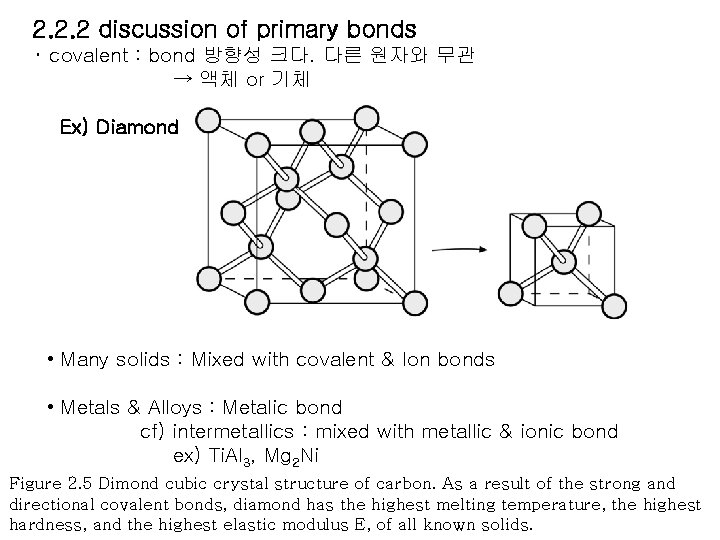
2. 2. 2 discussion of primary bonds ・ covalent : bond 방향성 크다. 다른 원자와 무관 → 액체 or 기체 Ex) Diamond • Many solids : Mixed with covalent & Ion bonds • Metals & Alloys : Metalic bond cf) intermetallics : mixed with metallic & ionic bond ex) Ti. Al 3, Mg 2 Ni Figure 2. 5 Dimond cubic crystal structure of carbon. As a result of the strong and directional covalent bonds, diamond has the highest melting temperature, the highest hardness, and the highest elastic modulus E, of all known solids.
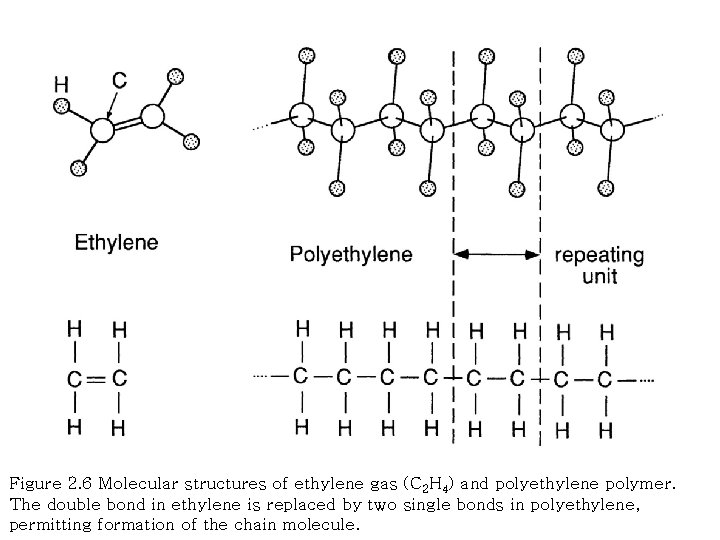
Figure 2. 6 Molecular structures of ethylene gas (C 2 H 4) and polyethylene polymer. The double bond in ethylene is replaced by two single bonds in polyethylene, permitting formation of the chain molecule.

2. 2. 3 secondary bonds ・ electrostatic dipole bond • permanent dipole bond ex) hydrogen bond - Water Figure 2. 7 Oxygen-to-hydrogen secondary bonds between water (H 2 O) molecules.
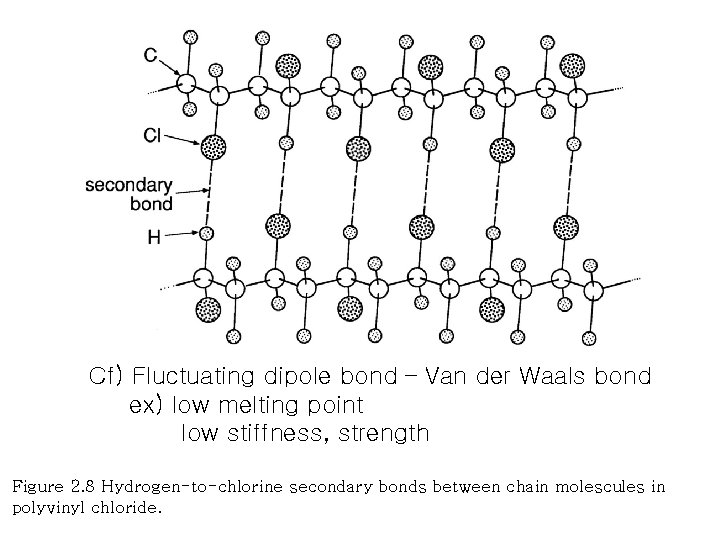
Cf) Fluctuating dipole bond – Van der Waals bond ex) low melting point low stiffness, strength Figure 2. 8 Hydrogen-to-chlorine secondary bonds between chain molescules in polyvinyl chloride.
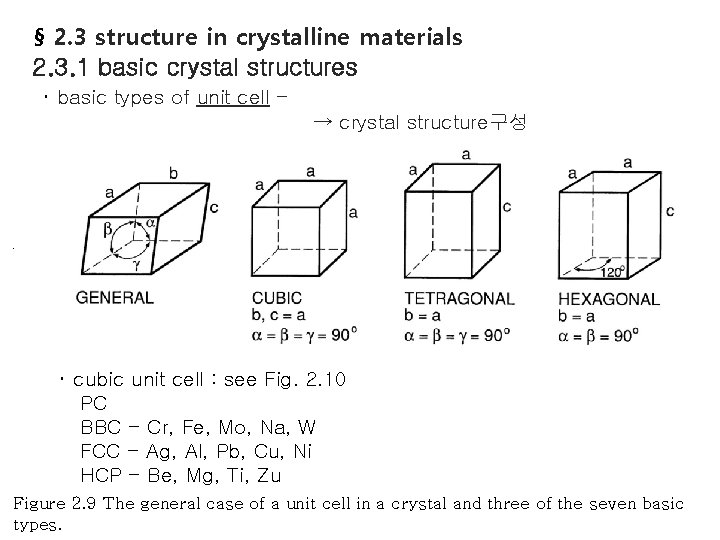
§ 2. 3 structure in crystalline materials 2. 3. 1 basic crystal structures ・ basic types of unit cell ・ → crystal structure구성 cubic unit cell : see Fig. 2. 10 PC BBC - Cr, Fe, Mo, Na, W FCC - Ag, Al, Pb, Cu, Ni HCP - Be, Mg, Ti, Zu Figure 2. 9 The general case of a unit cell in a crystal and three of the seven basic types.

Figure 2. 10 Foul crystal structures: primitive cubic(PC), body-centered cubic(BCC), face-centered cubic(FCC), and hexagonal close-packed (HCP) structures.
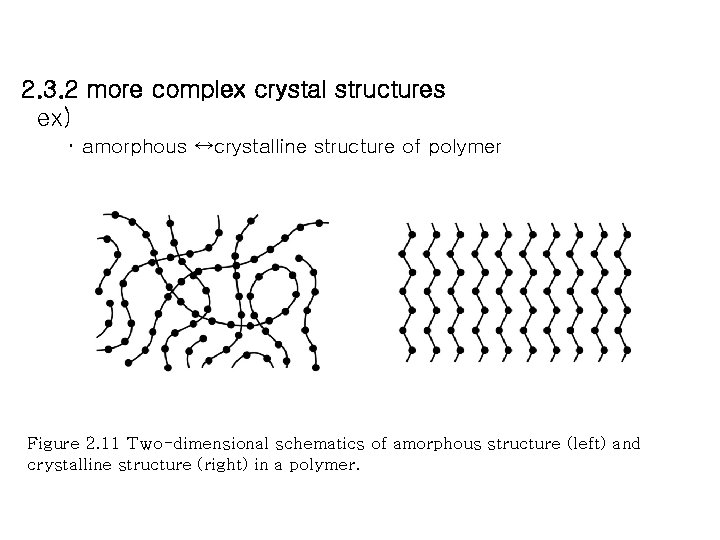
2. 3. 2 more complex crystal structures ex) ・ amorphous ↔crystalline structure of polymer Figure 2. 11 Two-dimensional schematics of amorphous structure (left) and crystalline structure (right) in a polymer.
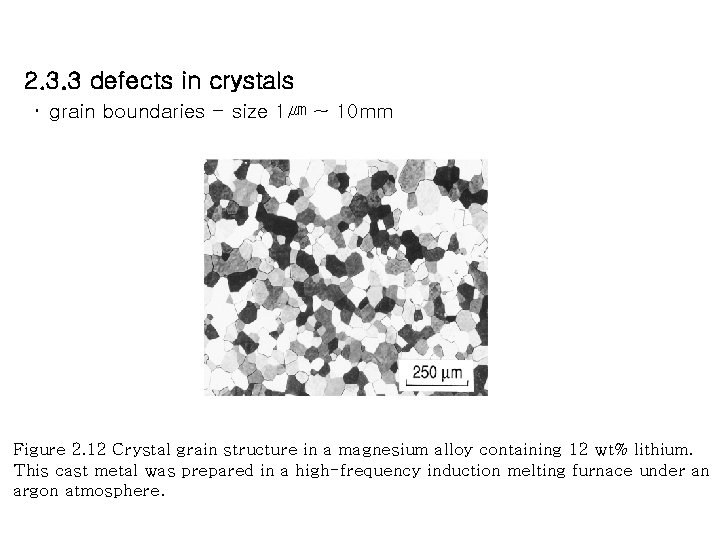
2. 3. 3 defects in crystals ・ grain boundaries - size 1㎛ ~ 10 mm Figure 2. 12 Crystal grain structure in a magnesium alloy containing 12 wt% lithium. This cast metal was prepared in a high-frequency induction melting furnace under an argon atmosphere.
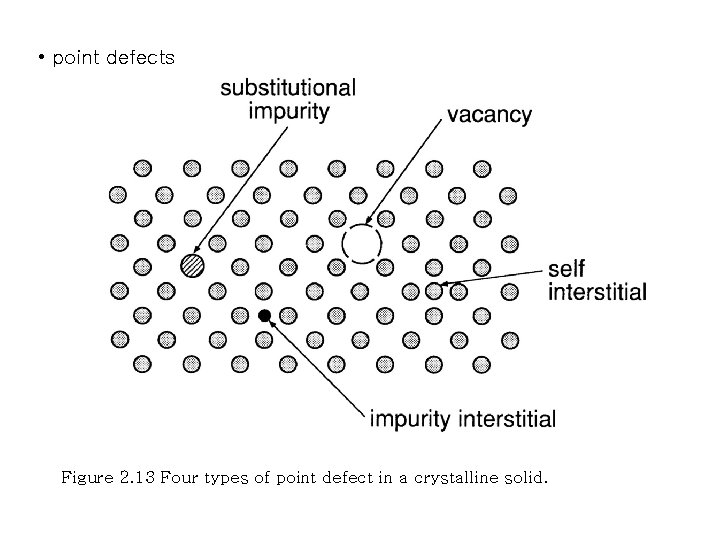
• point defects Figure 2. 13 Four types of point defect in a crystalline solid.
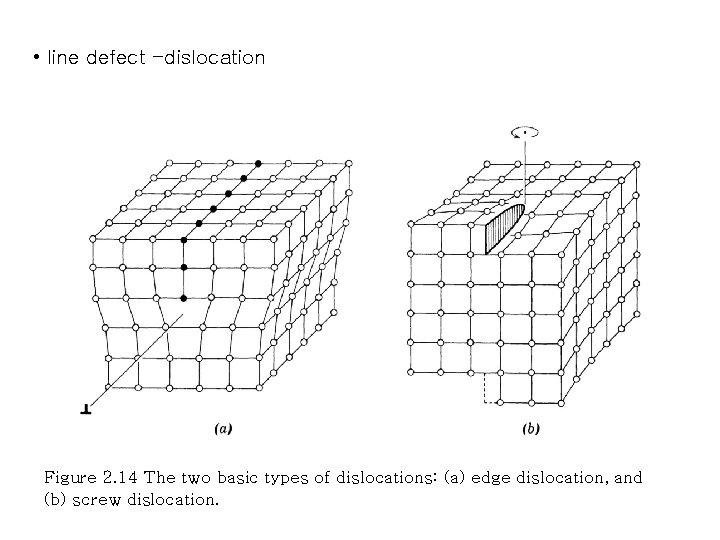
• line defect -dislocation Figure 2. 14 The two basic types of dislocations: (a) edge dislocation, and (b) screw dislocation.
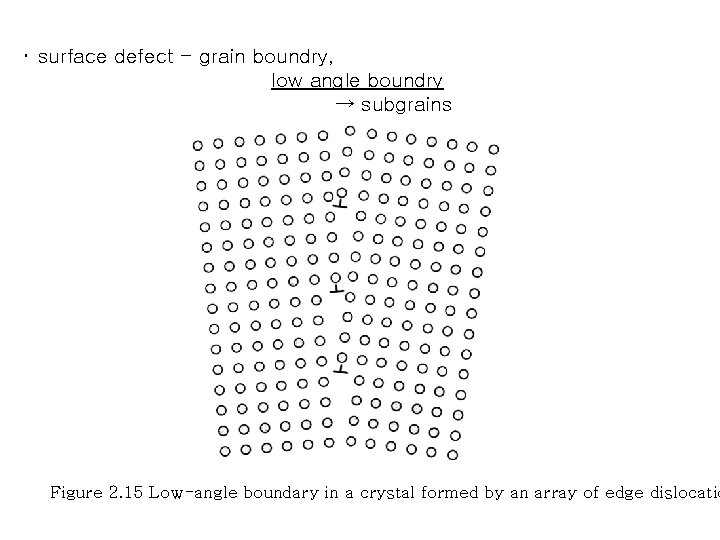
・ surface defect - grain boundry, low angle boundry → subgrains Figure 2. 15 Low-angle boundary in a crystal formed by an array of edge dislocatio
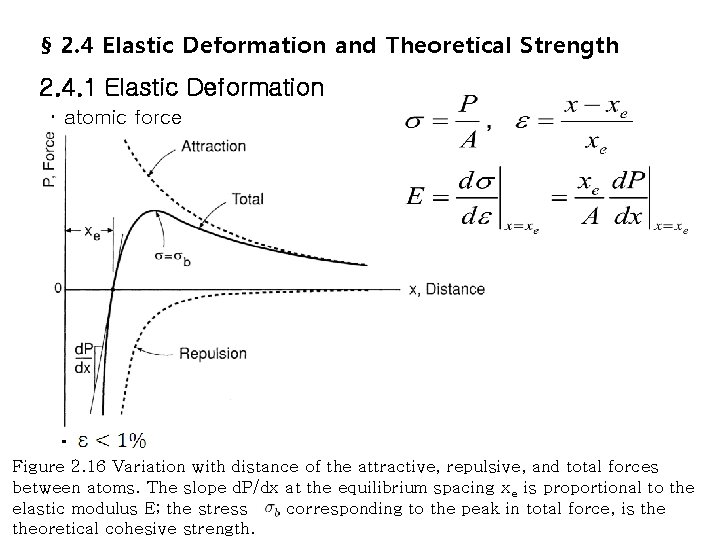
§ 2. 4 Elastic Deformation and Theoretical Strength 2. 4. 1 Elastic Deformation ・ atomic force ・ Figure 2. 16 Variation with distance of the attractive, repulsive, and total forces between atoms. The slope d. P/dx at the equilibrium spacing xe is proportional to the elastic modulus E; the stress , corresponding to the peak in total force, is theoretical cohesive strength.
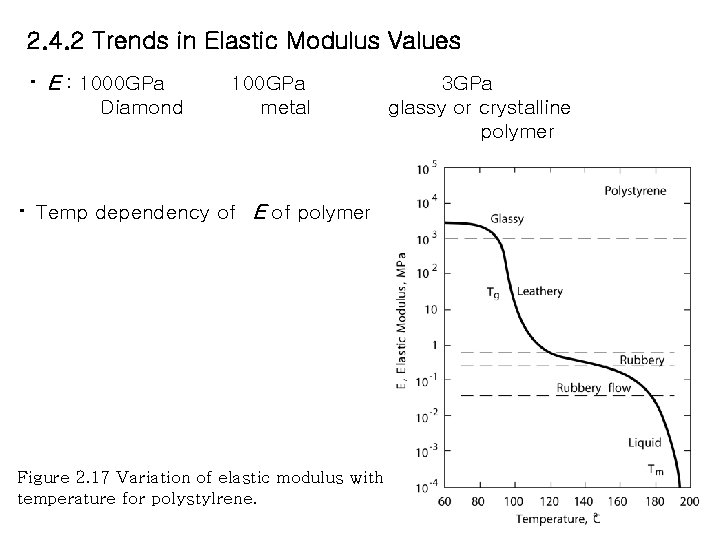
2. 4. 2 Trends in Elastic Modulus Values ・ E : 1000 GPa 100 GPa 3 GPa Diamond metal glassy or crystalline polymer ・ Temp dependency of E of polymer Figure 2. 17 Variation of elastic modulus with temperature for polystylrene.
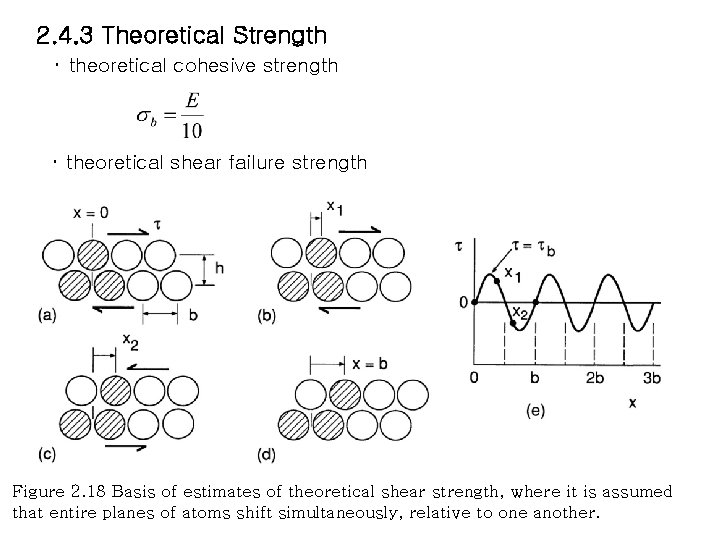
2. 4. 3 Theoretical Strength ・ theoretical cohesive strength ・ theoretical shear failure strength Figure 2. 18 Basis of estimates of theoretical shear strength, where it is assumed that entire planes of atoms shift simultaneously, relative to one another.
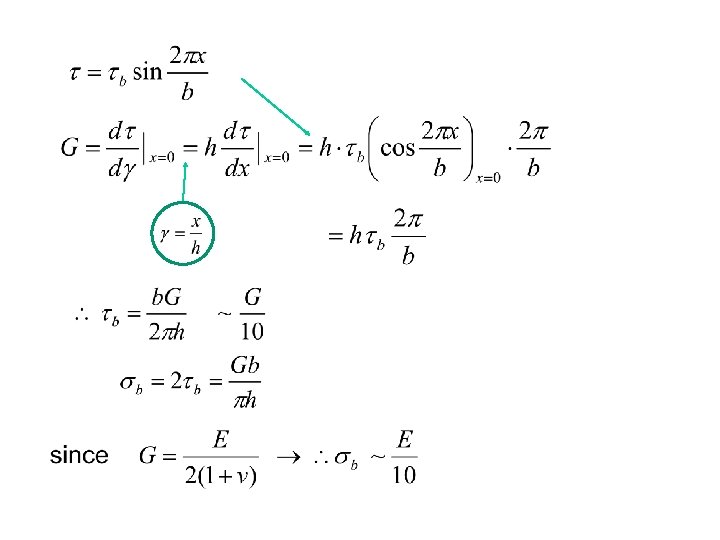
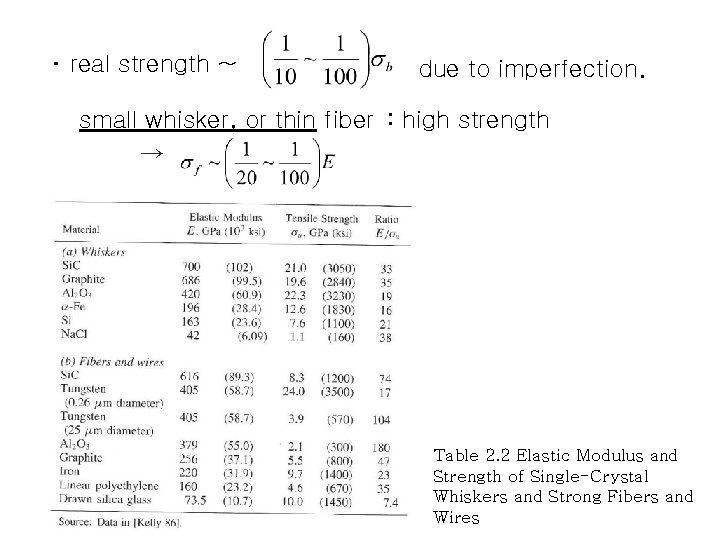
・ real strength ~ due to imperfection. small whisker, or thin fiber : high strength → Table 2. 2 Elastic Modulus and Strength of Single-Crystal Whiskers and Strong Fibers and Wires

§ 2. 5 Inelastic Deformation 2. 5. 1 Plastic Deformation by Dislocation Motion ・ BCC metal - ・ FCC, HCP ・ dislocation move -> slip (imperfect crystal ) Figure 2. 19 Shear deformation occurring in an incremental manner due to dislocation motion.
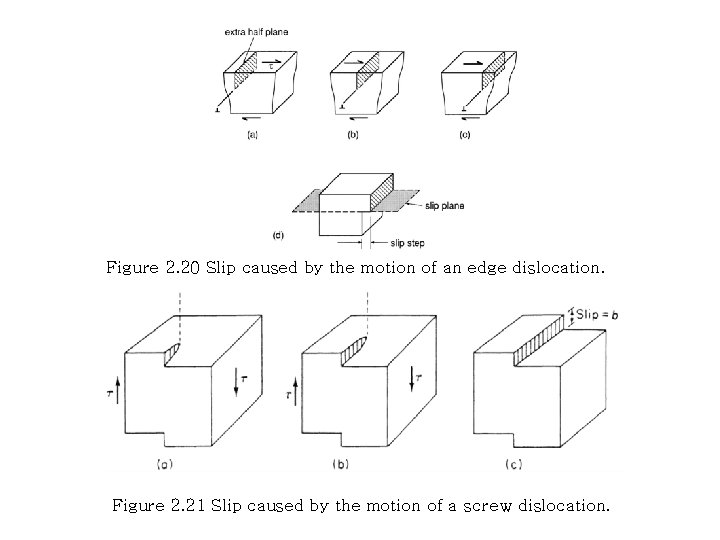
Figure 2. 20 Slip caused by the motion of an edge dislocation. Figure 2. 21 Slip caused by the motion of a screw dislocation.

・plastic deform concentrated in bands called “slip band” -> intense plastic shear deformation Figure 2. 22 Slip bands and slip steps caused by the motion of many dislocations resulting from cyclic loading of AISI 1010 steel.
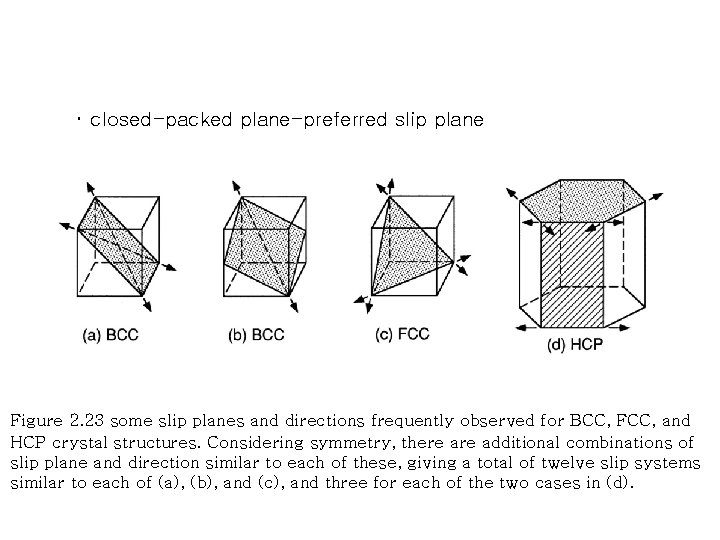
・ closed-packed plane-preferred slip plane Figure 2. 23 some slip planes and directions frequently observed for BCC, FCC, and HCP crystal structures. Considering symmetry, there additional combinations of slip plane and direction similar to each of these, giving a total of twelve slip systems similar to each of (a), (b), and (c), and three for each of the two cases in (d).
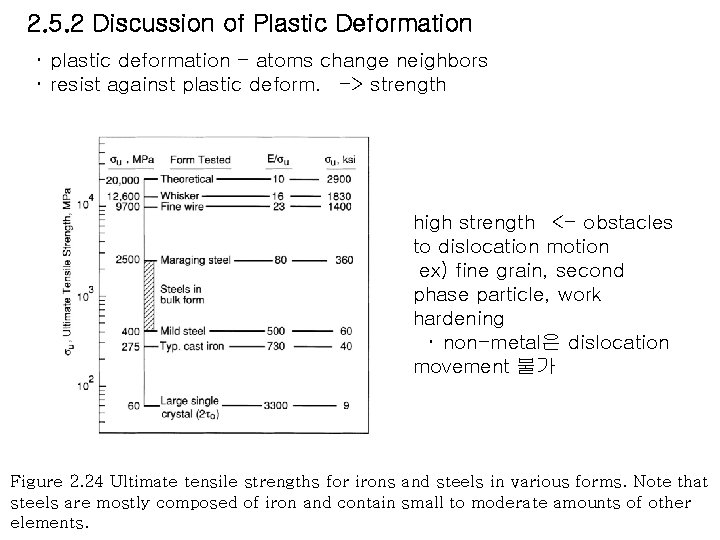
2. 5. 2 Discussion of Plastic Deformation ・ plastic deformation - atoms change neighbors ・ resist against plastic deform. -> strength high strength <- obstacles to dislocation motion ex) fine grain, second phase particle, work hardening ・ non-metal은 dislocation movement 불가 Figure 2. 24 Ultimate tensile strengths for irons and steels in various forms. Note that steels are mostly composed of iron and contain small to moderate amounts of other elements.

2. 5. 3 Creep Deformation ・ time dependent Figure 2. 25 Accumulation of creep strain with time under constant stress, and partial recovery after removal of the stress. ・ mechanism of creep of crystalline materials diffusion flow of vacancies. Figure 2. 26 Mechanism of creep by diffusion of vacancies within a crystal grain.

Other mechanisms 43하단 ~44상단 ・ 근처에서 creep 중요
- Slides: 45