Chapter 1 Fire Measurement and the SI System




- Slides: 28

Chapter 1 Fire Measurement and the SI System of Units

Objectives • Explain the importance of measurement in understanding fire behavior. • Name the basic SI units of measurement and covert between values in SI units and English units.

Objectives • Understand the precision of a measurement and the reduced precision used in estimations. • Explain the differences between mass and weight and among energy, heat, and enthalpy.

About Measurement • Key to understanding fire phenomena • Ask: – When fire started – How rapid the growth – How hot – How severe a threat

About Measurement • SI units—current international system: – Mass – Length – Time – Electrical current – Temperature

About Measurement • SI system adoption led to quantitative communication. • Precision limits exist according to measuring instruments. – Okay to estimate calculated value as needed

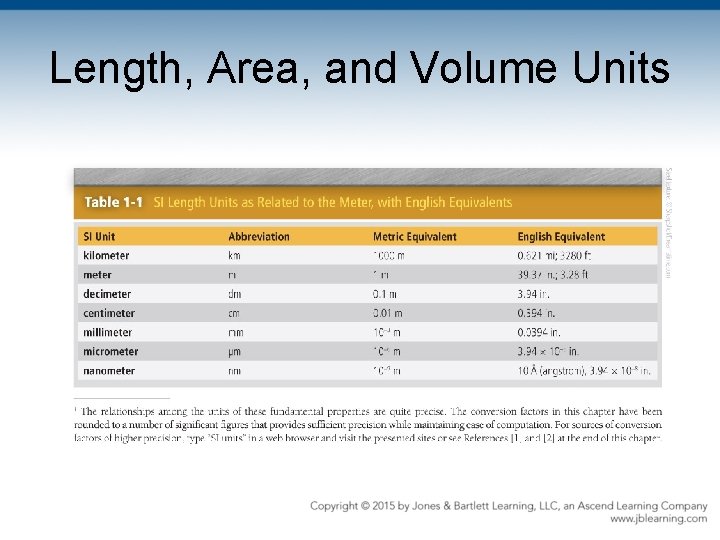
Length, Area, and Volume Units • Meter—basic SI unit of length – Length of path traveled by light in 1/299, 792, 458 of 1 second – SI dimensions In multiples of 1000: km, m, mm • Others: cm, dm

Length, Area, and Volume Units

Length, Area, and Volume Units • Area—two-dimensional – Rectangular flat surface: length of surface × width – Small areas: • SI: Square meters, square centimeters • English system: square inches, square feet

Length, Area, and Volume Units • Larger areas: – Metric system—hectares (ha) • 1 hectare = 10, 000 m 2 – English equivalent— 1 hectare = 2. 47 acres – Small areas: • SI: square meters, square centimeters • English system: square inches, square feet

Length, Area, and Volume Units • Fire dynamics calculations – Cross-sectional area of vent for flow through the vent – Area of hot surface for heat transferred to colder objects

Length, Area, and Volume Units • Volume—three-dimensional – Rectangular space—length × width × height – Cubic meters, cubic centimeters, etc. – Liter • Unit of liquid and gas volume • Equal to 1 cubic decimeter, 1000 cubic centimeters • Equal to 0. 264 U. S. gallon or 1. 056 quarts

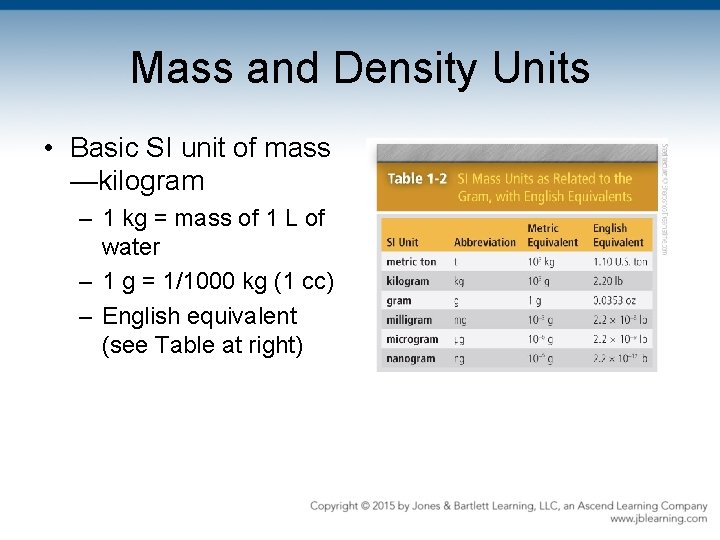
Mass and Density Units • Basic SI unit of mass —kilogram – 1 kg = mass of 1 L of water – 1 g = 1/1000 kg (1 cc) – English equivalent (see Table at right)

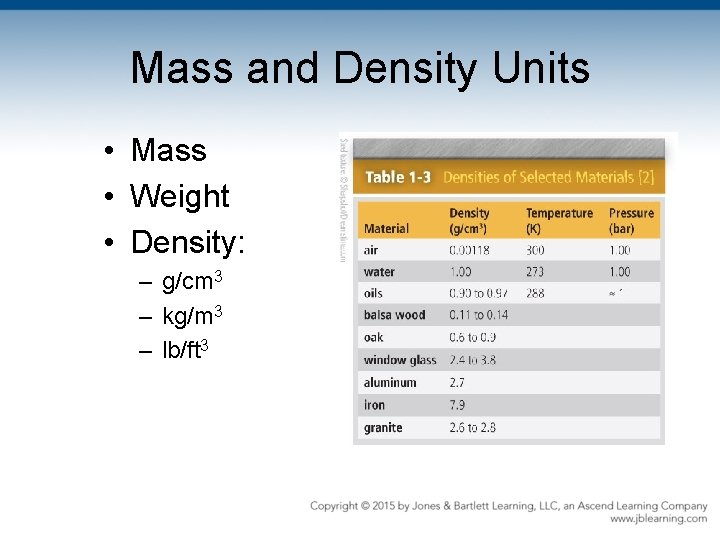
Mass and Density Units • Mass • Weight • Density: – g/cm 3 – kg/m 3 – lb/ft 3

Mass and Density Units • To denote relative prevalence of components: – Concentration: Component mass per unit volume – Volume fraction (gases) – Mass fraction

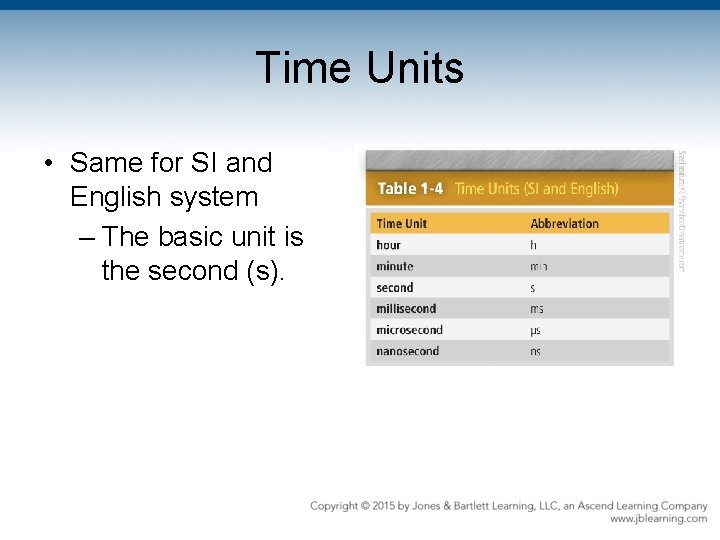
Time Units • Same for SI and English system – The basic unit is the second (s).

Time Units • Speed—rate at which an object is moving – m/s – km/h • Velocity—speed in a chosen direction • Acceleration—rate of change of speed

Force and Pressure Units • Basic unit of force in SI system—newton – Force needed to accelerate a mass of 1 kg at the rate of 1 m/s 2 – English system— 1 lb of force will accelerate 1 lb of mass at the rate of 32. 2 ft/s 2 – 1 newton = 0. 224 lb force

Force and Pressure Units • Pressure—force per unit area – Basic SI unit of pressure—pascal (Pa) – Common English units of pressure • Pounds per square inch (psi) • Manometers

Energy and Enthalpy Units • Fire generates energy; this increase: – Transfers heat to system – Raises pressure within volume – Increases temperature – Increases spread outside the room

Energy and Enthalpy Units • Basic SI unit of enthalpy, energy, heat— joule – Eithermal or mechanical energy • 1 joule = 0. 239 calorie (cal) • 4. 187 J = 1 cal • Cal = energy needed to heat 1 g water by 1 o. C

Energy and Enthalpy Units • Heat of combustion • Effective heat of combustion – Units for both—k. J/g – English unit for enthalpy • Foot-pounds (ft-lb) • British thermal units (Btu)

Energy and Enthalpy Units • Power units – Power—the rate at which enthalpy or energy is expended • SI units—watts (W) • English units – Horsepower – British thermal units (Btu)

Energy and Enthalpy Units • Temperature units – Temperature—measure of a substance’s warmth or coldness • SI system: – Celsius scale (o. C) – Kelvin scale (K)

Energy and Enthalpy Units • Celsius scale (at sea level): – Water freezes at 0 o. C. – Water boils at 100 o. C. – Lowest temperature – 273. 15 o. C • To convert: – From K to o. C • Subtract 273. 15 (273) – From o. C to K • Add 273

Energy and Enthalpy Units • English system—Fahrenheit scale (o. F) – Water freezes at 32 o. F. – Water boils at 212 o. F. – Conversions: • From o. F to o. C: (o. F – 32)/1. 8 = o. C • From o. C to o. F: 1. 8(o. C) + 32 = o. F

Conversion Factors • MKS system: – Length—meters (m) – Mass—kilograms (kg) – Time—seconds (s) • CGS system: – Length— centimeters (cm) – Mass—grams (g) – Time—seconds (s)

Summary • Ability to measure (quantify) is essential to understanding fire phenomena. • Basic measurements for fire phenomena are time, length, area, volume, mass, density, force, pressure, enthalpy and energy, power, and temperature. • There are multiple units for each of these measurements (metric and English). Metric units are most widely used worldwide; English units remain in use in the U. S. • Familiarity with various units and their interconversion can minimize the change of making a serious calculation error.