Chapter 1 Elements Introduction to the Periodic Table

Chapter 1 Elements
Introduction to the Periodic Table • Each square shows the name of one element and its symbol. • First letter – Always capitalized – First letter of the name of the element (some symbols are derived from the latin or german name of the element • Second letter (if there is one) – Never capitalized
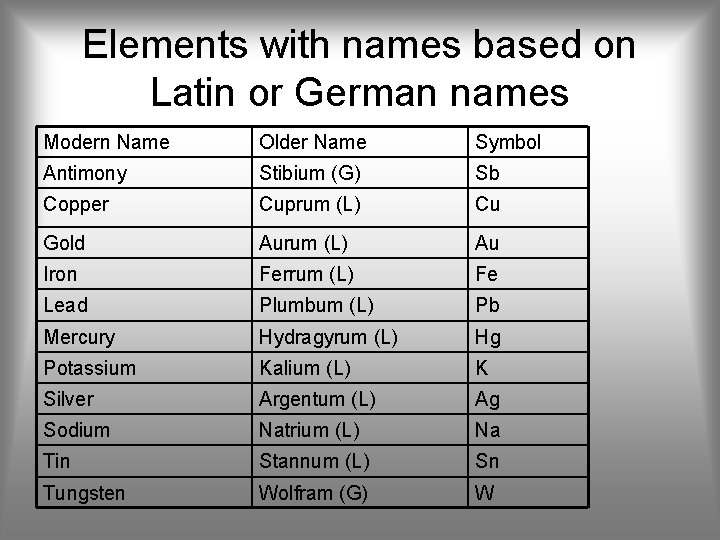
Elements with names based on Latin or German names Modern Name Older Name Symbol Antimony Stibium (G) Sb Copper Cuprum (L) Cu Gold Aurum (L) Au Iron Ferrum (L) Fe Lead Plumbum (L) Pb Mercury Hydragyrum (L) Hg Potassium Kalium (L) K Silver Argentum (L) Ag Sodium Natrium (L) Na Tin Stannum (L) Sn Tungsten Wolfram (G) W

Organization • Groups or families – Vertical columns – 18 – Each group contains elements with similar chemical properties • Periods – Horizontal rows – Physical and chemical properties change somewhat regularly across a period

Organization • Metals and Nonmetals – A stair-step line separates the metals from the nonmetals – Metalloids straddle the line • Lanthanide and Actinide Series – Two sets of elements that are placed under the periodic table to keep the table from being too wide – They fit into the table after elements 57 and 89
Types of Elements • Metals – left and center of table – Luster (shininess) – Conductivity – both electricity and heat – Malleability – can be hammered or rolled into thin sheets – Ductile – can be drawn into a fine wire – High tensile strength – resist breaking when pulled
Types of Elements • Nonmetals – Right of the table – Many are gases at room temperature – One (bromine) is a liquid – Some are solids (carbon, phosphorus, selenium, sulfur, iodine) • Solid nonmetals tend to be brittle rather than malleable and ductile – Poor conductors of heat and electricity
http: //borntoexplore. org/chemistry/metalloids. jpg Types of Elements • Metalloids – Found along stair-step line separating metals from nonmetals – Has some characteristics of metals and some of nonmetals – Solids at room temp – Less malleable than metals but not as brittle as nonmetals – Semiconductors of electricity

Types of Elements • Noble Gases – Group 18 at far right of table – Generally unreactive – Gases at room temperature http: //prl. dcu. ie/gallery-images/gallery_015. jpg
- Slides: 9