Chapter 1 Electronic Structure and Bonding Acids and
Chapter 1 Electronic Structure and Bonding Acids and Bases
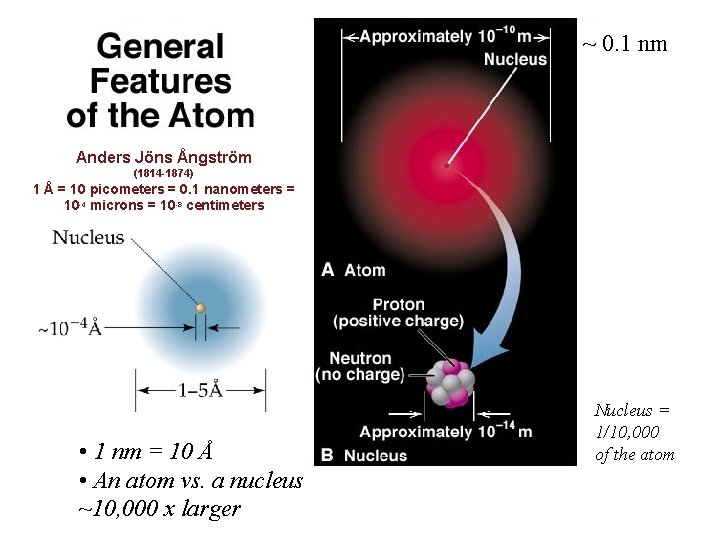
~ 0. 1 nm Anders Jöns Ångström (1814 -1874) 1 Å = 10 picometers = 0. 1 nanometers = 10 -4 microns = 10 -8 centimeters • 1 nm = 10 Å • An atom vs. a nucleus ~10, 000 x larger Nucleus = 1/10, 000 of the atom
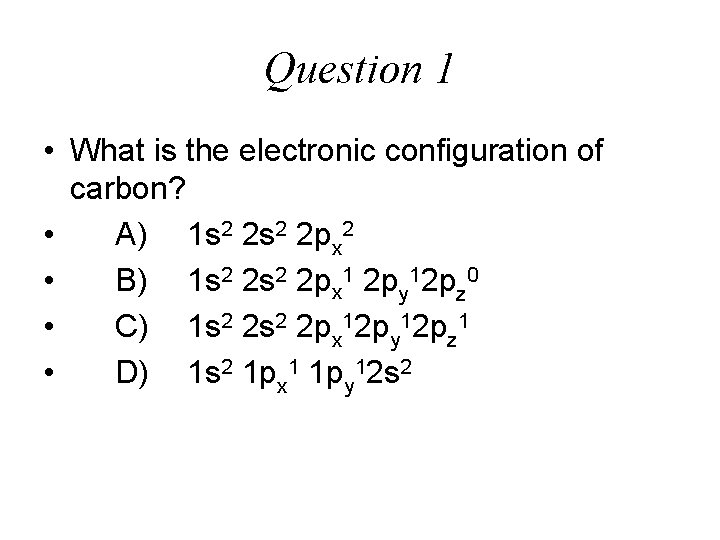
Question 1 • What is the electronic configuration of carbon? • A) 1 s 2 2 px 2 • B) 1 s 2 2 px 1 2 py 12 pz 0 • C) 1 s 2 2 px 12 py 12 pz 1 • D) 1 s 2 1 px 1 1 py 12 s 2
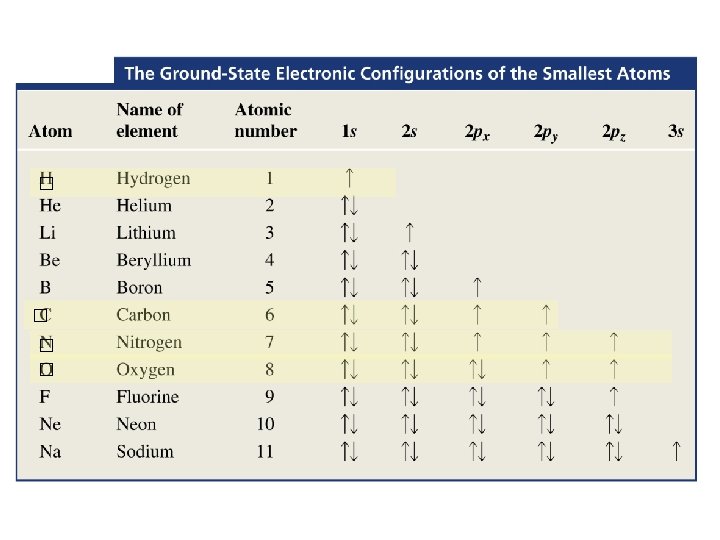
Electron Configurations Noble Gases and The Rule of Eight • When two nonmetals react to form a covalent bond: They share electrons to achieve a Noble gas electron configuration. • When a nonmetal and a metal react to form an ionic compound: Valence electrons of the metal are lost and the nonmetal gains these electrons.
G. N. Lewis Photo Bancroft Library, University of California/LBNL Image Library Footnote: G. N. Lewis, despite his insight and contributions to chemistry, was never awarded the Nobel prize. http: //chemconnections. org/organic/Movies%20 Org%20 Flash/Lewis. Dot. Structures. swf
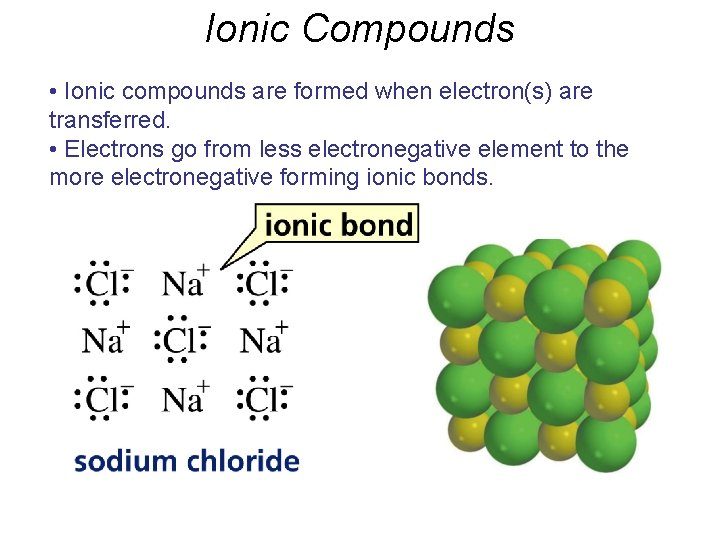
Ionic Compounds • Ionic compounds are formed when electron(s) are transferred. • Electrons go from less electronegative element to the more electronegative forming ionic bonds.
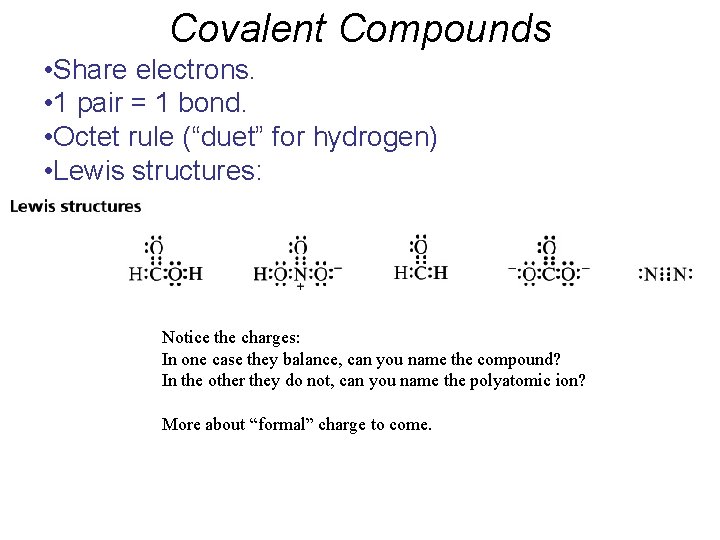
Covalent Compounds • Share electrons. • 1 pair = 1 bond. • Octet rule (“duet” for hydrogen) • Lewis structures: Notice the charges: In one case they balance, can you name the compound? In the other they do not, can you name the polyatomic ion? More about “formal” charge to come.
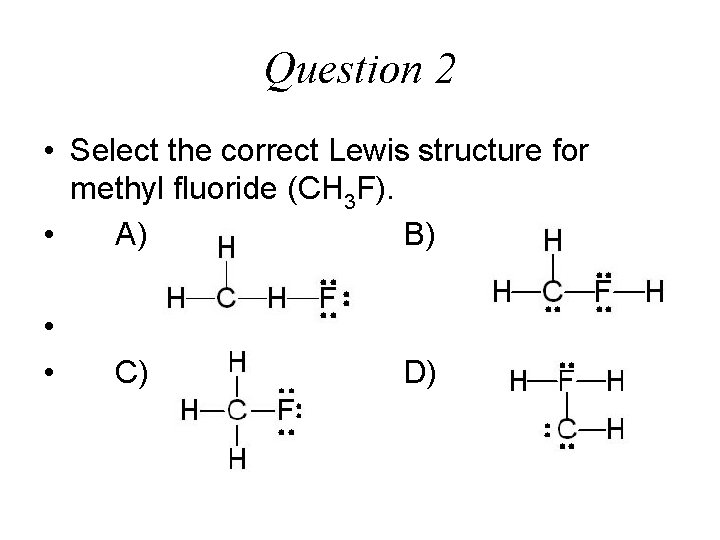
Question 2 • Select the correct Lewis structure for methyl fluoride (CH 3 F). • A) B) • • C) D)
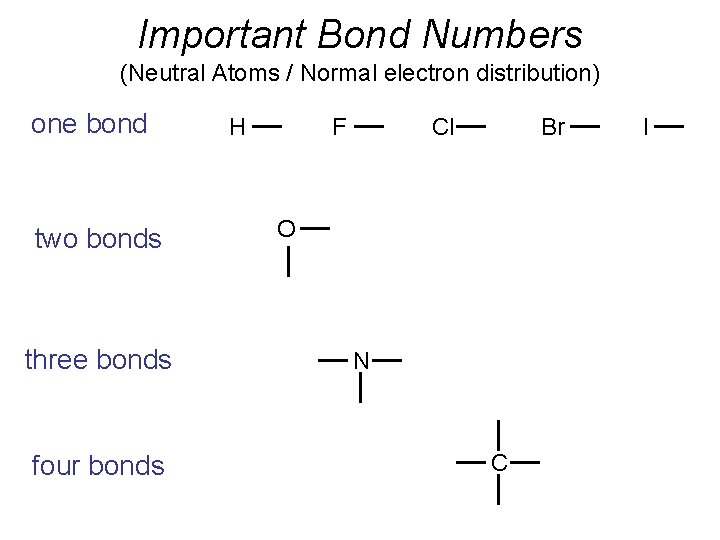
Important Bond Numbers (Neutral Atoms / Normal electron distribution) one bond two bonds three bonds four bonds H F Cl Br O N C I
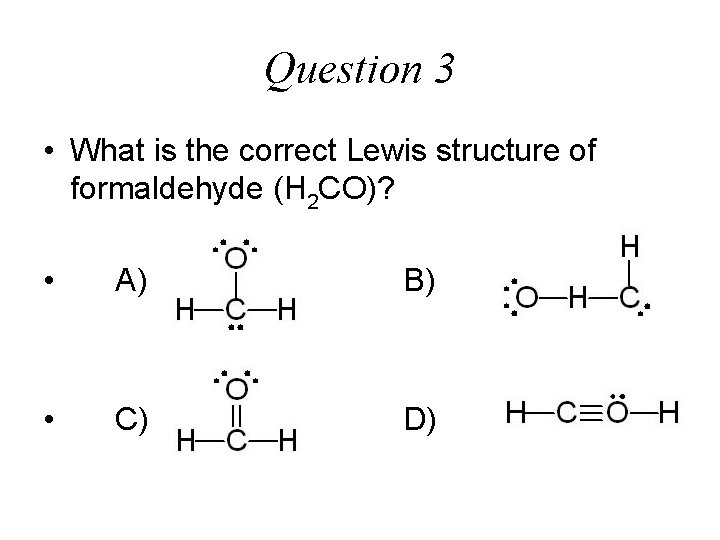
Question 3 • What is the correct Lewis structure of formaldehyde (H 2 CO)? • A) B) • C) D)

Question 4 • Which of the following contains a triple bond? • A) SO 2 • B) HCN • C) C 2 H 4 • D) NH 3

Formal Charge Formal charge is the charge of an atom in a Lewis structure which has a different than normal distribution of electrons.

Important Bond Numbers (Neutral Atoms / Normal electron distribution) one bond two bonds three bonds four bonds H F Cl Br O N C I

Important Bond Numbers (Neutral Atoms / Normal electron distribution)
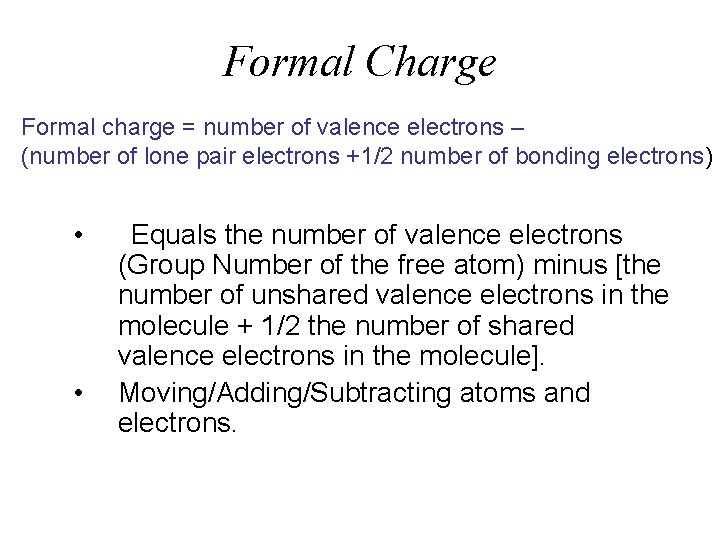
Formal Charge Formal charge = number of valence electrons – (number of lone pair electrons +1/2 number of bonding electrons) • • Equals the number of valence electrons (Group Number of the free atom) minus [the number of unshared valence electrons in the molecule + 1/2 the number of shared valence electrons in the molecule]. Moving/Adding/Subtracting atoms and electrons.

HNO 3 Nitric Acid
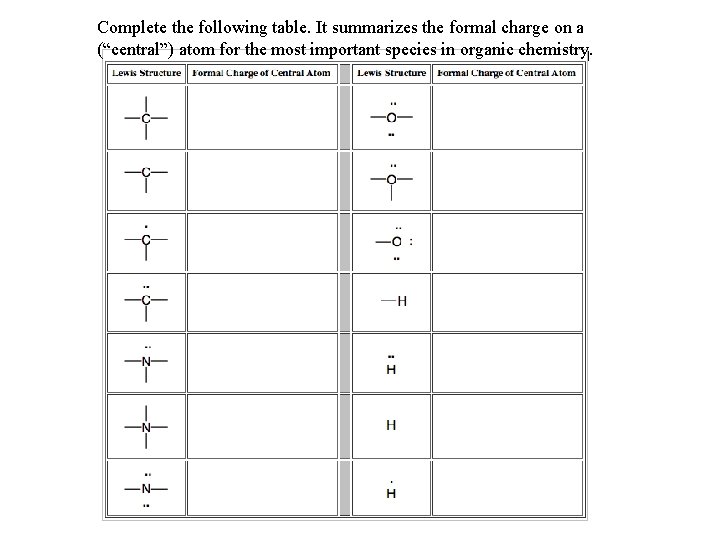
Complete the following table. It summarizes the formal charge on a (“central”) atom for the most important species in organic chemistry.
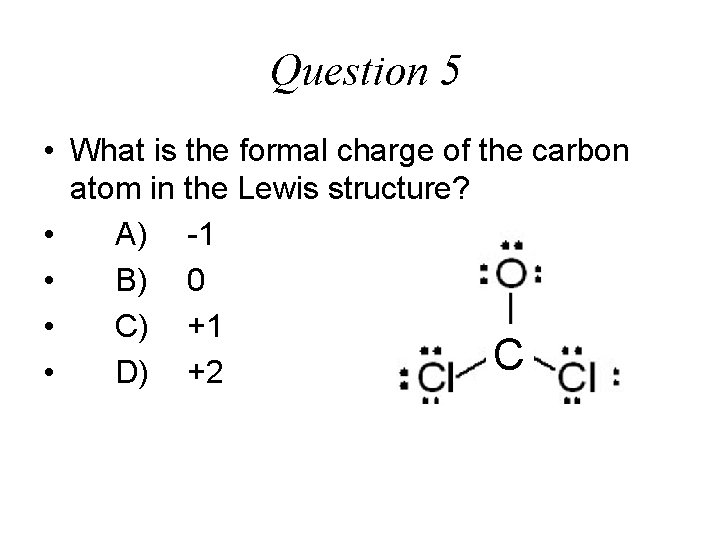
Question 5 • What is the formal charge of the carbon atom in the Lewis structure? • A) -1 • B) 0 • C) +1 C • D) +2
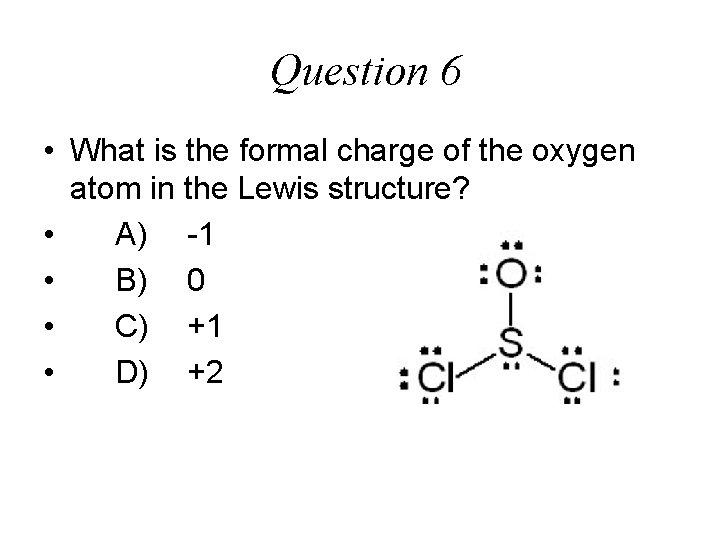
Question 6 • What is the formal charge of the oxygen atom in the Lewis structure? • A) -1 • B) 0 • C) +1 • D) +2

Resonance
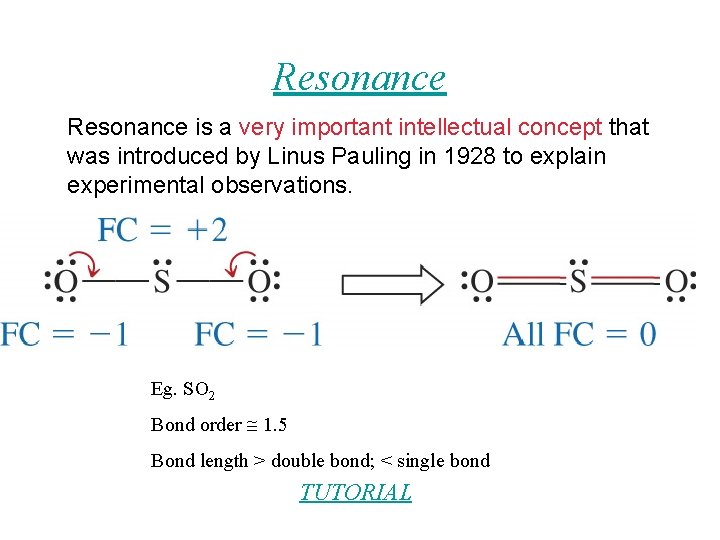
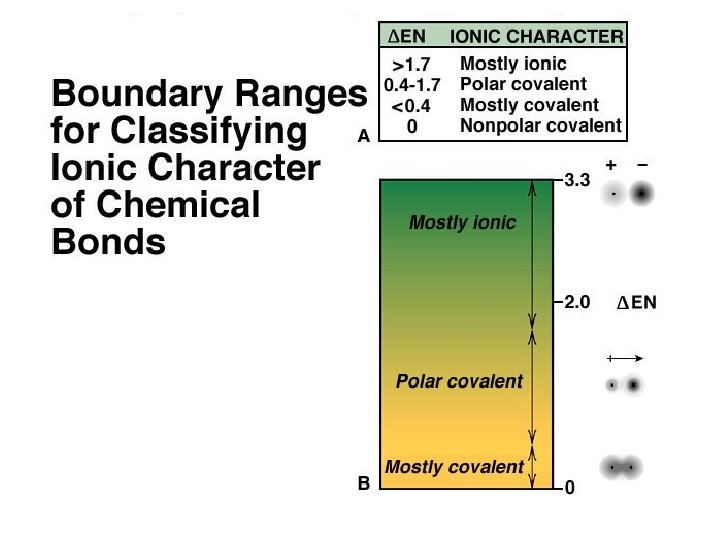
Resonance is a very important intellectual concept that was introduced by Linus Pauling in 1928 to explain experimental observations. Eg. SO 2 Bond order 1. 5 Bond length > double bond; < single bond TUTORIAL

Resonance • Two or more Lewis structures may be legitimately written for certain compounds (or ions) that have double bonds and/or free pairs of non-bonded electrons • It is a mental exercise in “pushing” or moving electrons. • Refer to Table 1. 6

Rules of Resonance Step 1: The atoms must stay in the same position. Atom connectivity is the same in all resonance structures. Only electrons move. NON-Example: The Lewis formulas below are not resonance forms. A hydrogen atom has changed position.

Rules of Resonance • Step 2: Each contributing structure must have the same total number of electrons and the same net charge. • Example: All structures have 18 electrons and a net charge of 0.

Rules of Resonance • Step 3: Calculate formal charges for each atom in each structure. • Example: None of the atoms possess a formal charge in this Lewis structure.
Rules of Resonance • • Step 4: Calculate formal charges for the second and third structures. Example: These structures have formal charges. NOTE: They are less favorable Lewis structures.
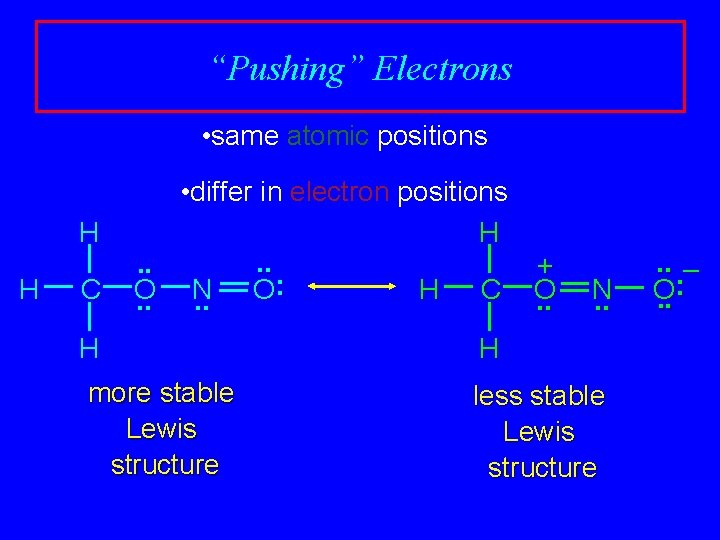
“Pushing” Electrons • same atomic positions H H C • differ in electron positions H. . + : O N O H C O. . . H more stable Lewis structure N. . H less stable Lewis structure . . – : O. .
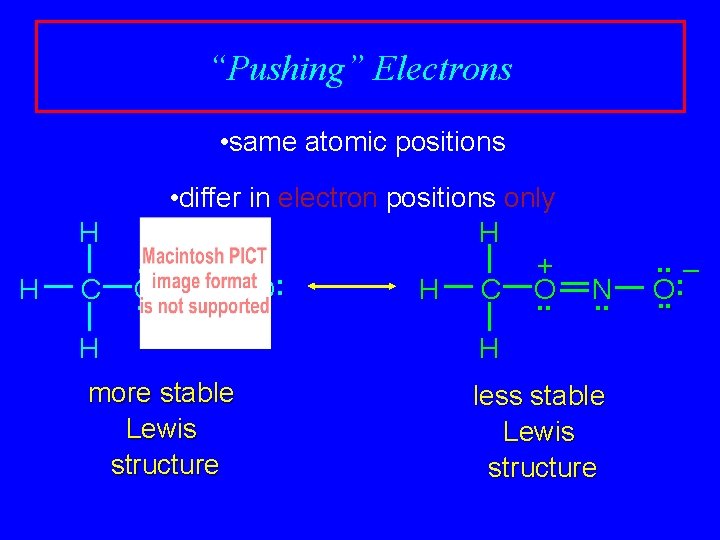
“Pushing” Electrons • same atomic positions H H C • differ in electron positions only H. . + : O N O H C O. . . H more stable Lewis structure N. . H less stable Lewis structure . . – : O. .
Why use Resonance Structures? • Delocalization of electrons and charges between two or more atoms helps explain energetic stability and chemical reactivity. • Electrons in a single Lewis structure are insufficient to show electron delocalization. • A composite of all resonance forms more accurately depicts electron distribution. (HYBRID) NOTE: Resonance forms are not always evenly weighted. Some forms are better than others.

Resonance Example • Ozone (O 3) –Lewis structure of ozone shows one double bond and one single bond • • • O • Expect: one short bond and one long bond Reality: bonds are of equal length (128 pm) + O • • • – O • • •
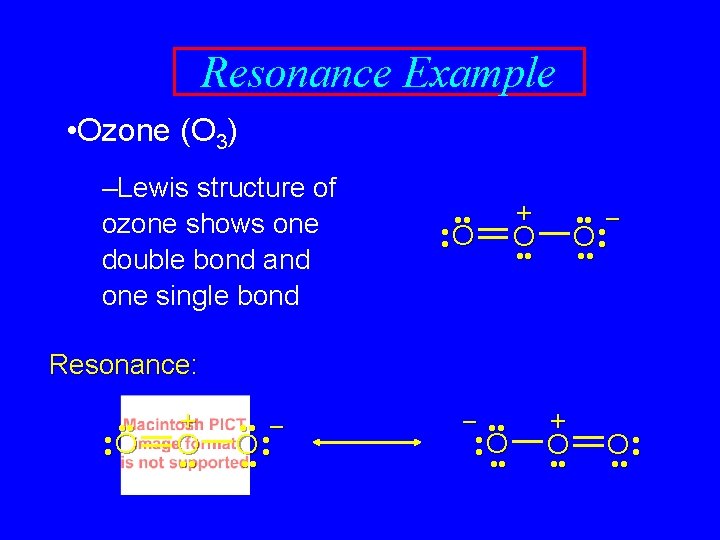
Resonance Example • Ozone (O 3) –Lewis structure of ozone shows one double bond and one single bond • • • O • + O • • • – O • • • Resonance: • • • O • + O • • • – O • • • – • • • O • • • + O • •
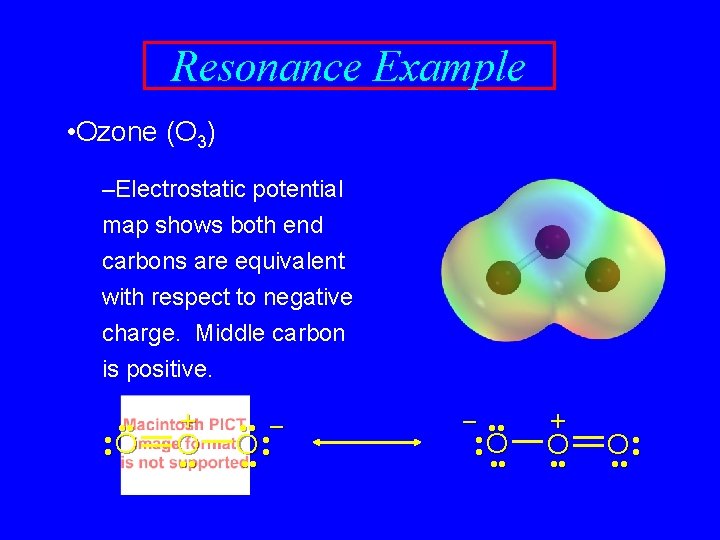
Resonance Example • Ozone (O 3) –Electrostatic potential map shows both end carbons are equivalent with respect to negative charge. Middle carbon is positive. • • • O • + O • • • – O • • • – • • • O • • • + O • •

Detailed Resonance Examples
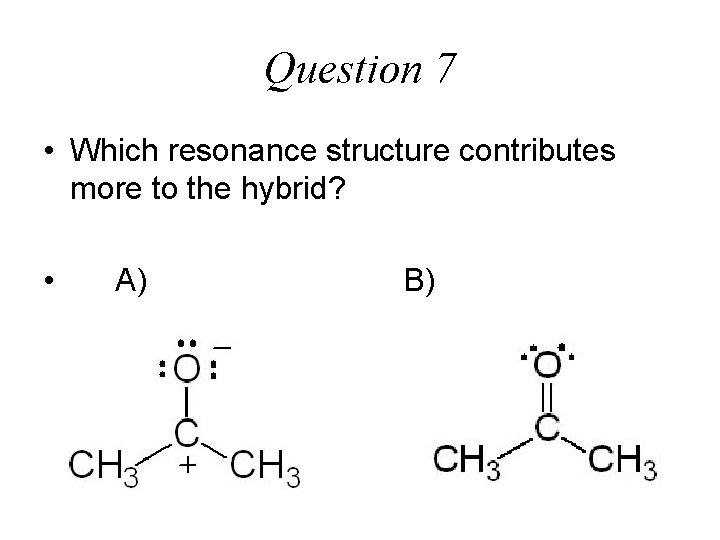
Question 7 • Which resonance structure contributes more to the hybrid? • A) B)
VSEPR Model Valence Shell Electron Pair Repulsion
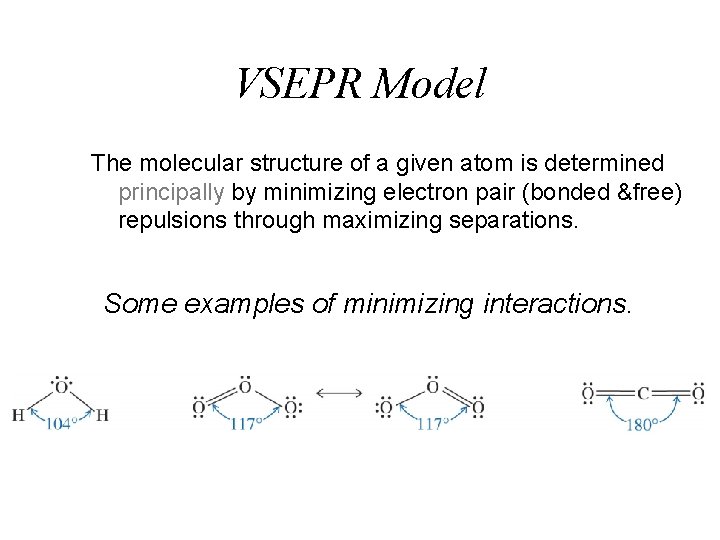
VSEPR Model The molecular structure of a given atom is determined principally by minimizing electron pair (bonded &free) repulsions through maximizing separations. Some examples of minimizing interactions.
Predicting a VSEPR Structure • 1. Draw Lewis structure. • 2. Put pairs as far apart as possible. • 3. Determine positions of atoms from the way electron pairs are shared. • 4. Determine the name of molecular structure from positions of the atoms.
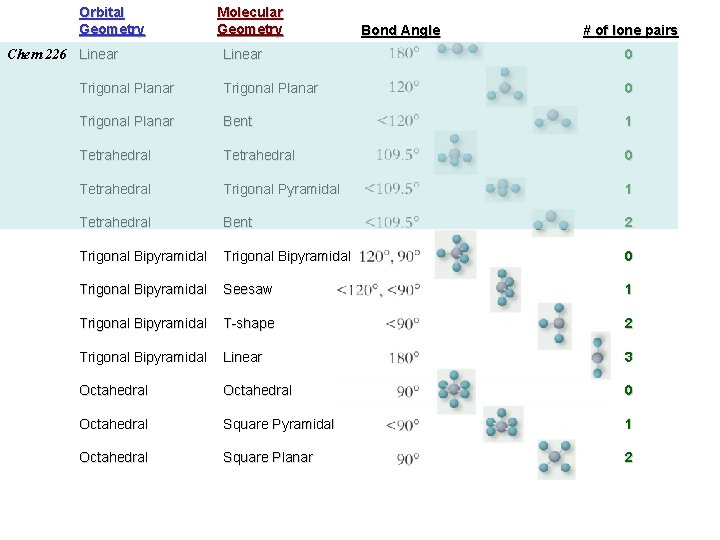
Orbital Geometry Chem 226 Linear Molecular Geometry Bond Angle # of lone pairs Linear 0 Trigonal Planar Bent 1 Tetrahedral 0 Tetrahedral Trigonal Pyramidal 1 Tetrahedral Bent 2 Trigonal Bipyramidal 0 Trigonal Bipyramidal Seesaw 1 Trigonal Bipyramidal T-shape 2 Trigonal Bipyramidal Linear 3 Octahedral 0 Octahedral Square Pyramidal 1 Octahedral Square Planar 2
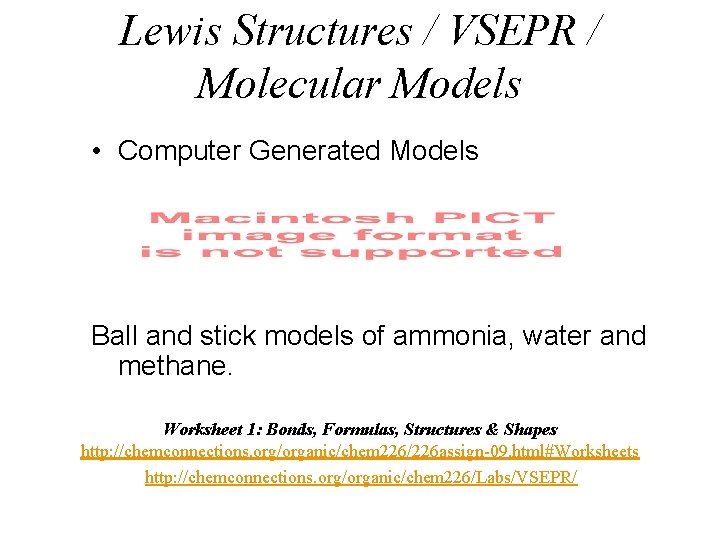
Lewis Structures / VSEPR / Molecular Models • Computer Generated Models Ball and stick models of ammonia, water and methane. Worksheet 1: Bonds, Formulas, Structures & Shapes http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets http: //chemconnections. org/organic/chem 226/Labs/VSEPR/
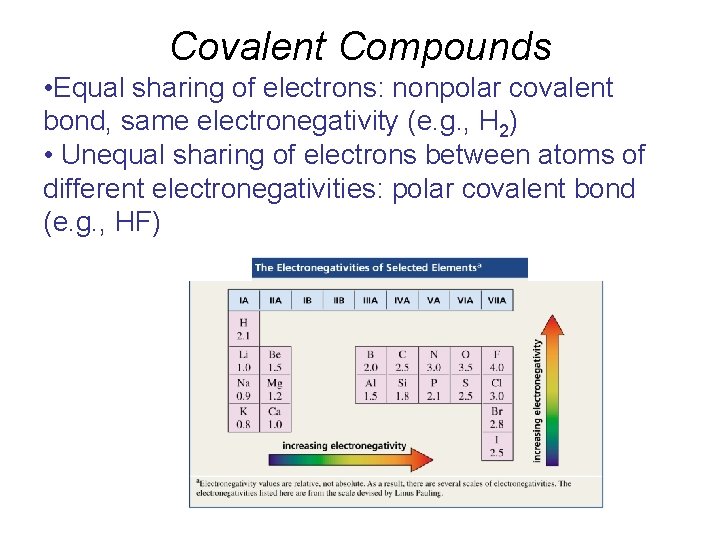
Covalent Compounds • Equal sharing of electrons: nonpolar covalent bond, same electronegativity (e. g. , H 2) • Unequal sharing of electrons between atoms of different electronegativities: polar covalent bond (e. g. , HF)
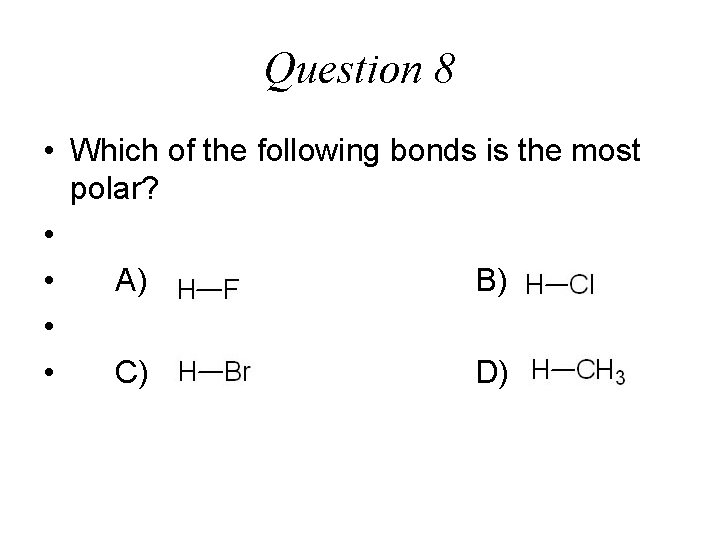
Question 8 • Which of the following bonds is the most polar? • • A) B) • • C) D)
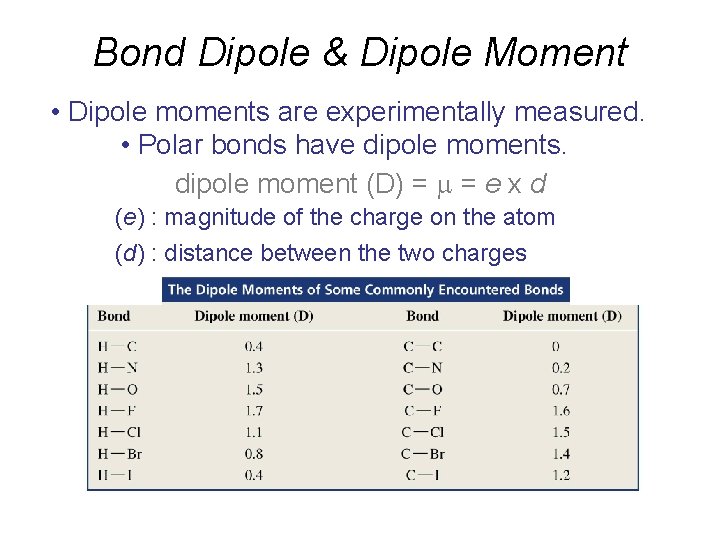
Bond Dipole & Dipole Moment • Dipole moments are experimentally measured. • Polar bonds have dipole moments. dipole moment (D) = = e x d (e) : magnitude of the charge on the atom (d) : distance between the two charges
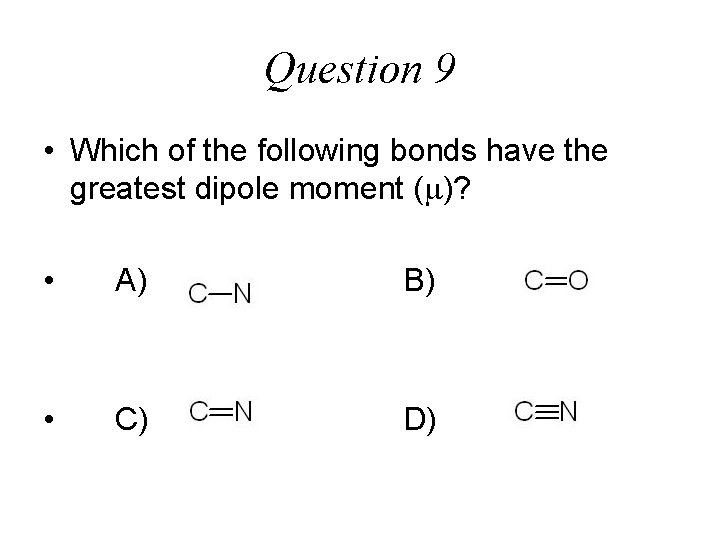
Question 9 • Which of the following bonds have the greatest dipole moment ( )? • A) B) • C) D)
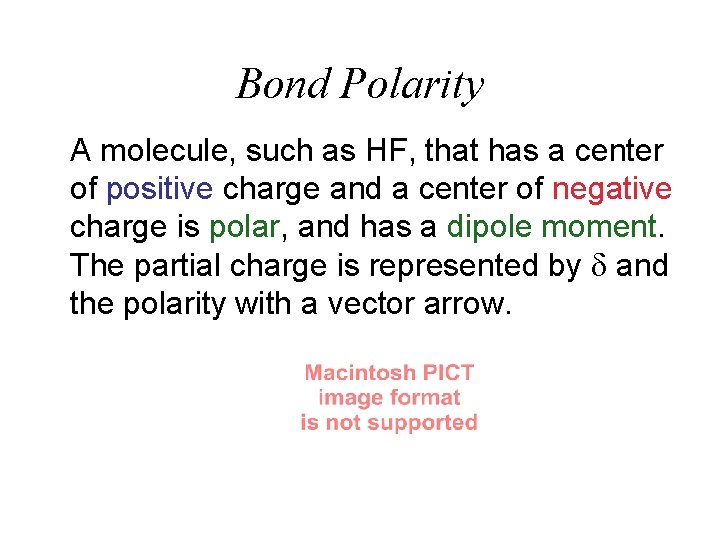
Bond Polarity A molecule, such as HF, that has a center of positive charge and a center of negative charge is polar, and has a dipole moment. The partial charge is represented by and the polarity with a vector arrow.

Question 10 • In which of the compounds below is the + for H the greatest? • A) CH 4 • B) NH 3 • C) Si. H 4 • D) H 2 O

Question 11 • In which of the following is oxygen the positive end of the bond dipole? • A) O-F • B) O-N • C) O-S • D) O-H
- Slides: 49