Chapter 1 Chemistry What is Chemistry A whole

































- Slides: 39

Chapter 1 Chemistry: What is Chemistry (A whole lot of fun!)

Objectives • • • Describe chemistry Classify and describe the types of matter Describe physical and chemical properties Describe physical and chemical changes Recognize clues a chemical change has occurred

What is Chemistry? • The study of the matter, its composition, properties, and the changes it undergoes. • Applied Chemistry is the using chemistry to attain certain goals, in fields like medicine, agriculture, and manufacturing • Pure chemistry gathers knowledge for knowledge sake

Types of Chemistry • Analytical Chemistry studies composition of substances. • Inorganic Chemistry substances without carbon • Organic Chemistry compounds containing carbon • Biochemistry- Chemistry of living things • Physical Chemistry studies behavior of substances

Chemistry is • A natural science with a language and its own vocabulary. • A way of thinking.

What is Matter? • Matter is anything that takes up space and has mass. • Mass is the amount of matter in an object. – Mass is resistance to change in motion along a smooth and level surface. – Volume – measure of 3 D space

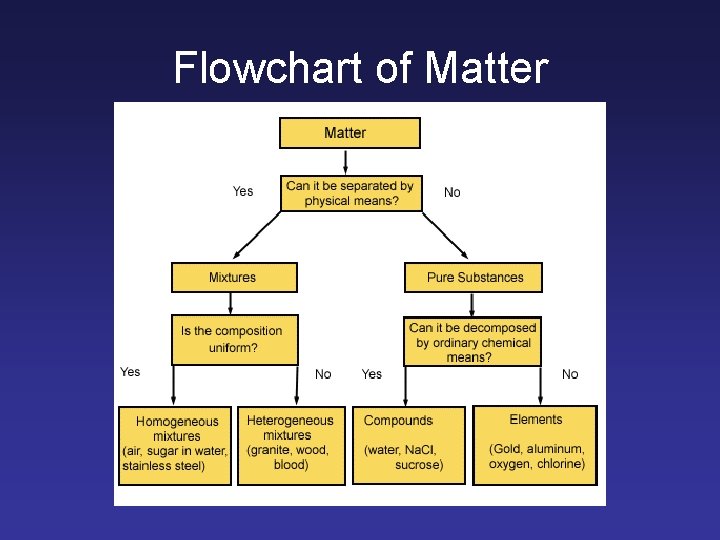
Types of Matter • Pure Substance- a particular kind of matter - pure • Mixture- more than one kind of matter

Substances • • Elements- simplest kind of matter Cannot be broken down All one kind of atom. Compounds are substances that can be broken down by chemical methods • When they are broken down, the pieces have completely different properties than the compound. • Made of molecules- two or more atoms

Atoms • The smallest unit of an element that maintains the properties of that element.


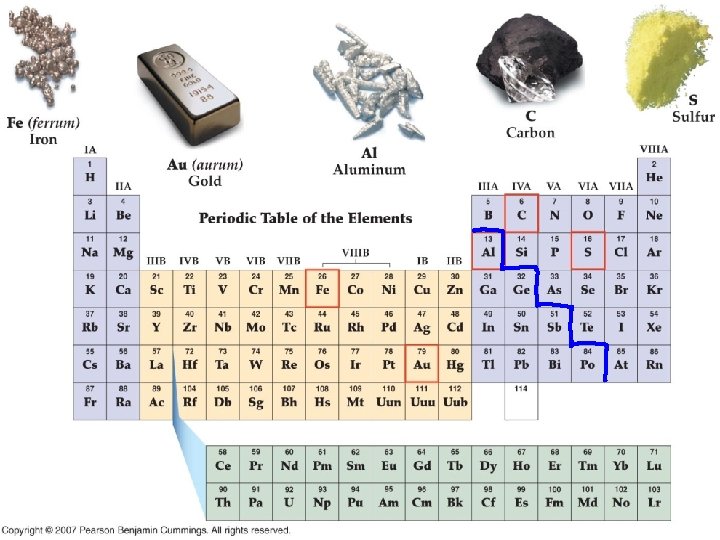
Elements • • • A pure substance made of only one kind of atom Currently 118 elements 90 natural 28 synthetic Found on periodic table Identified by 1, 2, or 3 letter chemical symbol

Chemical symbols • There are 118 elements • First letter always capitalized second and third never • Don’t need to memorize • Some are from Latin or other languages H= Hydrogen Au= Gold Uub=Ununbibium

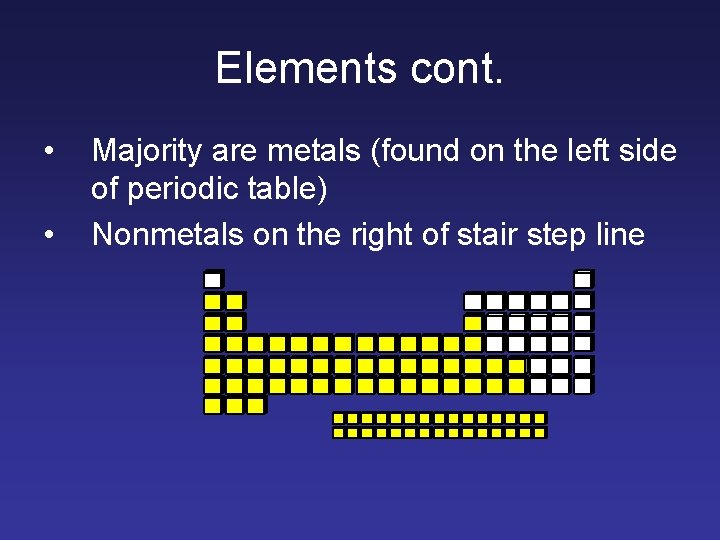
Elements cont. • • Majority are metals (found on the left side of periodic table) Nonmetals on the right of stair step line

Compounds • • • A substance that is made from the atoms of two or more elements that are chemically bonded Chemical Formula – Indicates type and number atoms in a compound Ex. H 2 O = 2 Hydrogen atoms and 1 Oxygen atom

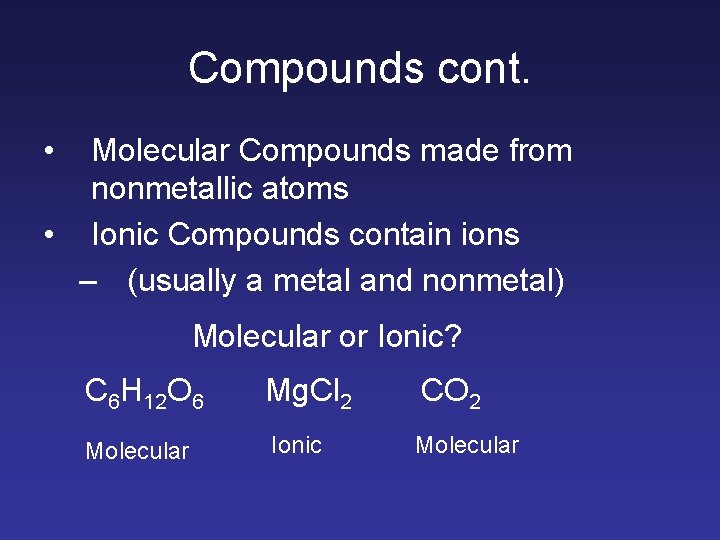
Compounds cont. • Molecular Compounds made from nonmetallic atoms • Ionic Compounds contain ions – (usually a metal and nonmetal) Molecular or Ionic? C 6 H 12 O 6 Mg. Cl 2 CO 2 Molecular Ionic Molecular

Metals • • • Luster Good conductor of heat and electricity Malleability Ductility High tensile strength

Nonmetals • • • Many nonmetals are gases at room temperature Solid nonmetals tend to be brittle Poor conductors of heat and electricity

Mixtures • A blend of two or more kinds of matter, each of which retains its own identity and properties • The components of mixtures can usually be separated through physical means – filtration, distillation, chromatography

Filtration • Uses a filter to separate substances – Solid from a liquid


Distillation • Separates based on boiling points – liquids


Chromatography • Separates based on polarity – What? !? !? ! • We’ll get to that later -Liquids or gases

Heterogeneous Mixtures • Called suspensions and colloids • Not uniform throughout – Ex. Chocolate chip cookie, gravel, soil

Homogeneous Mixture • • Called solutions Mixtures that are uniform throughout – Kool-aid, air, gold ring

Solutions • Like all mixtures, they keep the properties of the components. • Can be separated by physical means • Not easily separated- can be separated.

Solutions • Homogeneous mixture – Mixed molecule by molecule – Can occur between any state of matter. • Solid in liquid- Kool-aid • Liquid in liquid- antifreeze • Gas in gas- air • Solid in solid - brass • Liquid in gas- water vapor

Flowchart of Matter

Which is it? Mixture Compound Element

Physical Properties • A property that can be observed and measured without changing the substance. – Ex. melting point, boiling point, density, hardness, color, odor • Freezing Point vs. Melting Point?

States of matter • Solid- matter that can not flow and has definite volume. • Liquid- definite volume but takes the shape of its container (flows). • Gas- a substance without definite volume or shape and can flow.

Physical Change • A change that changes appearances, without changing the composition. 1. Freezing – liquid to solid 2. Melting – solid to liquid 3. Boiling – liquid to vapor 4. Condensation – vapor to liquid 5. Sublimation – solid to vapor 6. Deposition – vapor to solid

Chemical Properties • Relates to a substances ability to undergo changes that transform it into different substances – Ex. combust, oxidize, neutralize

Chemical Change • A change in which one or more substances are converted into different substances Ex. combustion, oxidation, neutralization

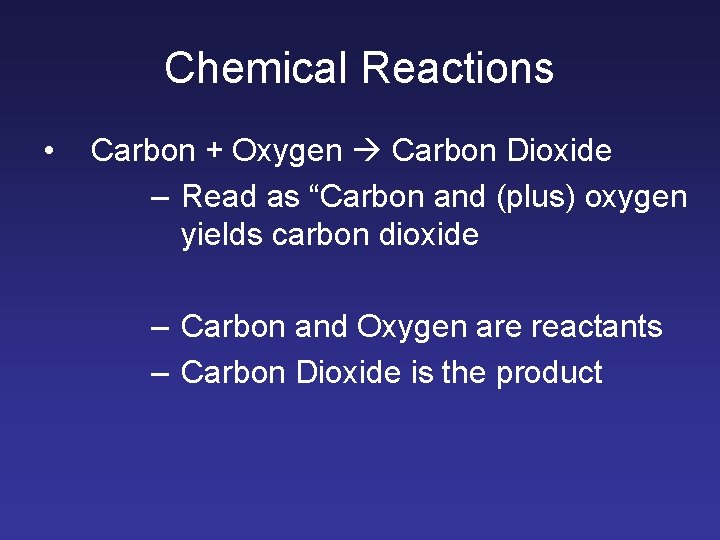
Chemical Reactions • Carbon + Oxygen Carbon Dioxide – Read as “Carbon and (plus) oxygen yields carbon dioxide – Carbon and Oxygen are reactants – Carbon Dioxide is the product

Chemical Reactions • When one or more substances are changed into new substances. – Reactants- stuff you start with – Products- What you make • NEW PROPERTIES • Not easily reversed

Indications of a chemical reaction • • • Energy absorbed or released Color change Gas is released Light given off Precipitate- solid that separates from solution p. 255 in text

Example

Homework • Page 21 #’s 25, 31, 34, 53, 54, 56, 68