CHAPTER 1 CHEMICAL REACTIONS Section 1 Forming New















- Slides: 24

CHAPTER 1: CHEMICAL REACTIONS

Section 1: Forming New Substances Chemical Changes are responsible for leaf coloring Chemical Reactions Process of one or more substance undergoes change to produce one or more different substances New substances had different chemical and/or physical characheristics

Clues to Chemical Reactions Gas Formation Solid Formation (Precipitate) Color Change Energy Change Breaking and Making Bonds New Substance Formed because: bonds break, atoms are rearranged, and new bonds form

Chemical Formulas Chemical Symbol-short-hand for identifying elements Chemical Formula-short-hand notation for a compound or diatomic element H 2 O 2 indicates 2 hydrogen Subscript—smaller/lower number No subscript indicates 1 atom of the element

Examples: O 2 C 6 H 12 O 6 2 Oxygen atoms 6 Carbon, 12 Hydrogen, 6 Oxygen

Writing Formulas for Covalent Compounds Usually made from two nonmetals Name can help write the formula Prefix often tells the # of atoms Mono-1 Di-2 Tri-3 Tetra-4 Penta-5 Hexa-6 Hepta-7 Octa-8 Nona-9 Deca-10

Writing Formulas for Ionic Compounds Likely to contain name of metal and nonmetal Overall charge must be zero—must balance with subscripts Na. Cl Mg. Cl 2

Chemical Equations Shorthand description of a chemical reaction using chemical symbols Understood throughout the world—no translation needed

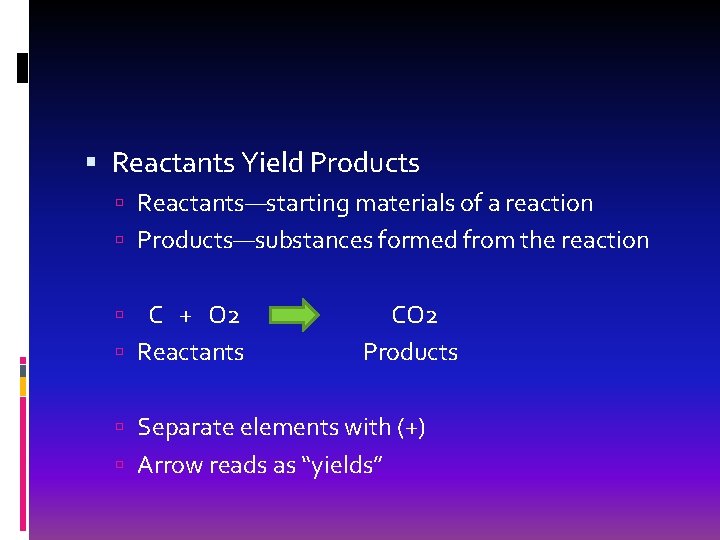
Reactants Yield Products Reactants—starting materials of a reaction Products—substances formed from the reaction C + O 2 Reactants CO 2 Products Separate elements with (+) Arrow reads as “yields”

Accuracy is important Diatomic elements: H 2, N 2, O 2, Fl 2, Cl 2, Br 2 , I 2 Inaccuracies can misrepresent information/characteristics CO 2—carbon dioxide/harmless gas CO—carbon monoxide/poisonous gas Co—Cobalt/grey metal

An Equation Must Be Balanced Atoms are not gained or lost in a reaction Number of reactant atoms must equal the number of product atoms

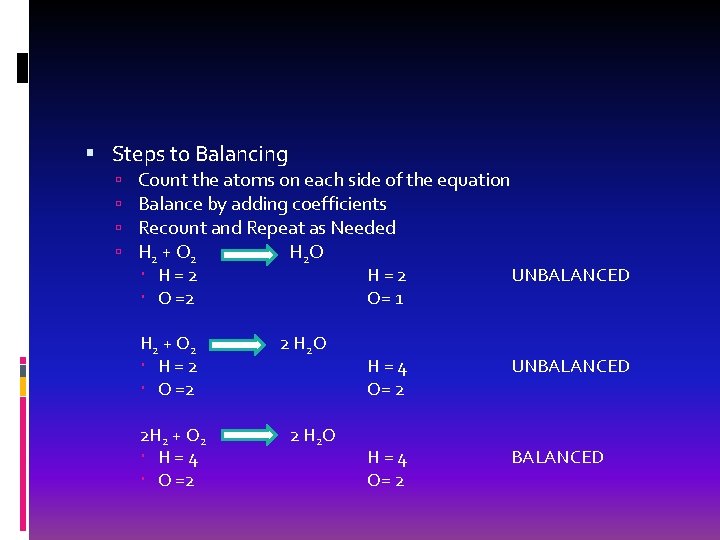
How to Balance an Equation Coefficients: number in front of a chemical symbol/formula To find the number of atoms: Multiply the coefficient by the subscript 2 CO 2 2 Carbon, 4 Oxygen

Steps to Balancing Count the atoms on each side of the equation Balance by adding coefficients Recount and Repeat as Needed H 2 + O 2 H 2 O H=2 UNBALANCED O =2 O= 1 H 2 + O 2 H=2 O =2 2 H 2 + O 2 H=4 O =2 2 H 2 O H=4 O= 2 UNBALANCED H=4 O= 2 BALANCED

Mass Is Conserved—It’s A Law! Law of Conservation of Mass: mass is neither created nor destroyed in an ordinary chemical or physical change Same number of each type of atom are found on each side of the equation

Section 2: Types of Chemical Reactions 4 Categories of Reactions: Synthesis, Decomposition, Single-Replacement, and Double Replacement Reactions

Synthesis Reactions Two or more substances combine to form a single substance 2 Na + Cl 2 2 Na. Cl

Decomposition Reaction Single compound breaks down to form two or more simple substances Reverse of synthesis reaction H 2 CO 3 H 2 O + CO 2

Single-Replacement Reaction One element takes the place of another element in a reaction Some elements are more reactive than others More reactive elements replace less reactive elements 2 N + 2 HCl 2 NCl 2 + H 2

Double-Replacement Reactions Ions in two compounds switch position Often a gas or precipitate is formed Na. Cl + Ag. F Na. F + Ag. Cl

Section 3: Energy and Rate of Chemical Reactions Every Reaction Involves Energy Bonds break with absorbed energy Bonds form with energy release Energy is Released in Exothermic Reactions Energy removed as light, electrical, or heat Energy is Absorbed in Endothermic Reactions Energy added—listed on left of equation

Section 3: Energy and Rate of Chemical Reactions Energy is Conserved—It’s a Law! Energy is neither created nor destroyed Activation Energy Gets a Reaction Started Activation Energy: minimum amount of energy needed for a substance to react Exothermic—energy released as a product supplies the activation energy to continue the reaction Endothermic—energy is needed to be supplied to keep the reaction going

Factors Affecting Rates of Reactions—How rapidly the reaction takes place Temperature Concentration Surface Area Presence of Catalyst or Inhibitor

Temperature Increase temperature increases rate Particles move faster and collide Concentration: Amount of substance dissolved in another Greater concentration generally increases the rate More substances are closer together and collide

Surface Area Increasing Area Speeds Rate Grinding particles to powder exposes more of reactants and more collide Catalysts and Inhibitors Catalysts: substances that speed up reactions without permanently being changed Inhibitor: substance that slows down or stops a chemical reaction