Chapter 1 Chapter 1 Introduction to Science Science




























- Slides: 41

Chapter 1


Chapter 1 Introduction to Science �Science involves observation and basic rules. �Science requires investigation, planned experimenting, observation, and extensive testing of results.

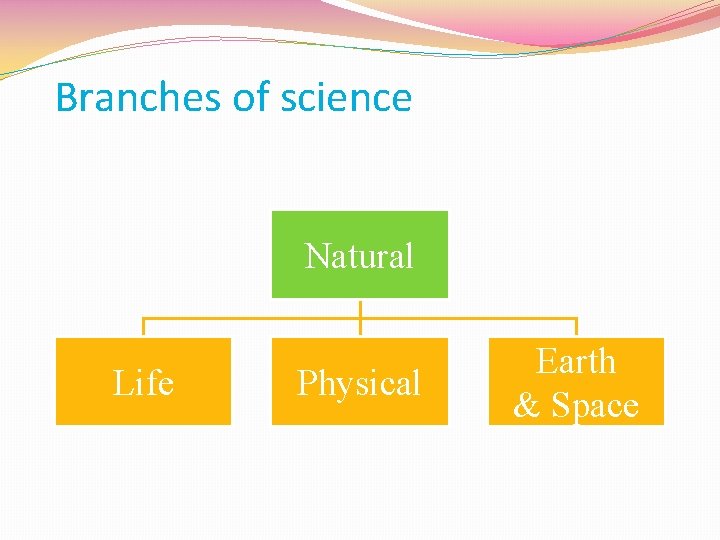
Branches of science � Two main branches are social and natural. � We will be learning about Natural Science

Branches of science Natural Life Physical Earth & Space

Science and Technology �Pure Science : the constant search for new knowledge. �Applied Science: take the work of pure scientist and look for practical applications. Technology, Engineering



Theory �Most logical explanation that has been tested by repeated observation. �Must explain observation simply and clearly �Experiments that illustrate a theory must be repeatable �Must be able to make predictions from your theory

Theories � Many theories change over time. �new discoveries are always being made. � Ex. Big Bang Theory


Law � A summary or statement of an observed natural event. � REMEBER, they do not explain the natural phenomenon.

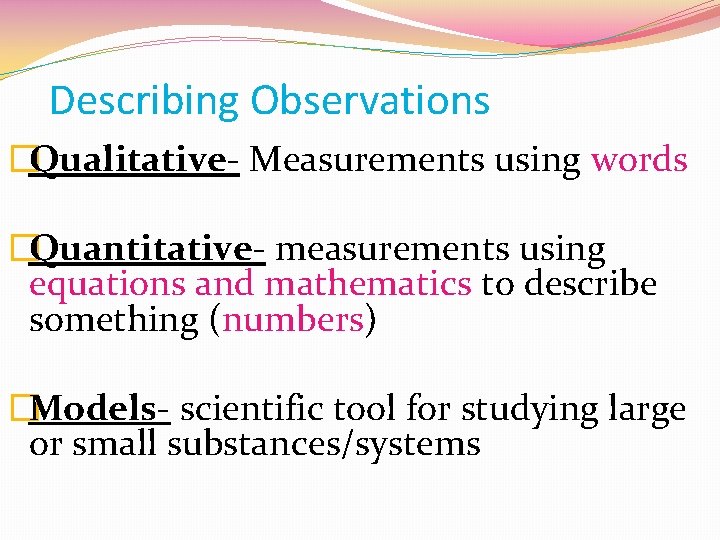
Describing Observations �Qualitative- Measurements using words �Quantitative- measurements using equations and mathematics to describe something (numbers) �Models- scientific tool for studying large or small substances/systems

Ch. 1. 2 The Scientific Method �This is an organized way of solving a problem or critical thinking. �Made up of several steps.


Practice! �Use the Scientific Method! �Observation �Questions/Problems �Create a hypothesis!

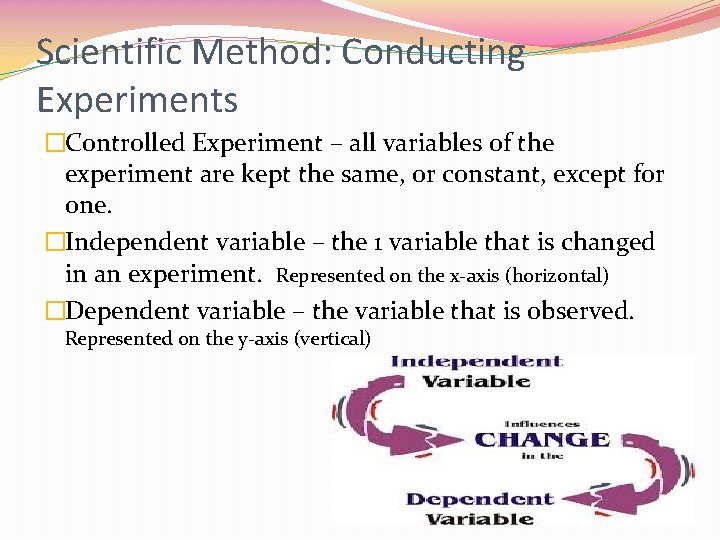
Scientific Method: Conducting Experiments �Controlled Experiment – all variables of the experiment are kept the same, or constant, except for one. �Independent variable – the 1 variable that is changed in an experiment. Represented on the x-axis (horizontal) �Dependent variable – the variable that is observed. Represented on the y-axis (vertical)

Ch 1. 3 Organizing Data �Graphs- a way for scientists to easily read and study data and information. �Line, bar, and pie graphs �Each graph has its own particular value when presenting info.

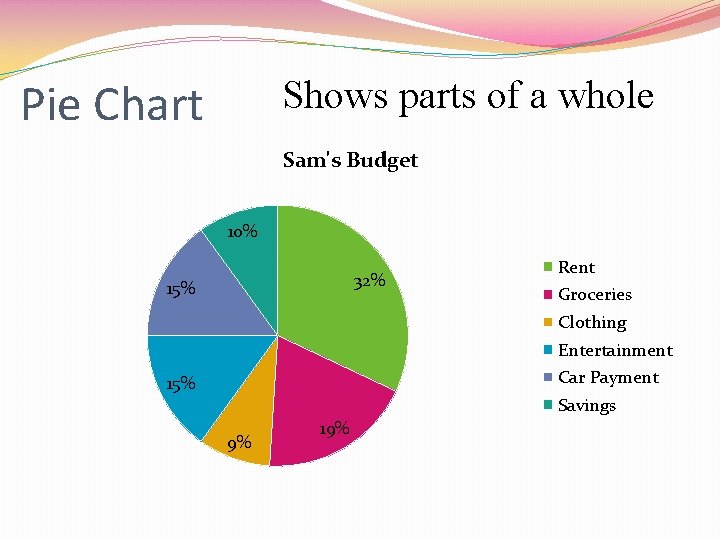
Pie Chart Shows parts of a whole Sam's Budget 10% 32% 15% Rent Groceries Clothing Entertainment Car Payment 15% Savings 9% 19%

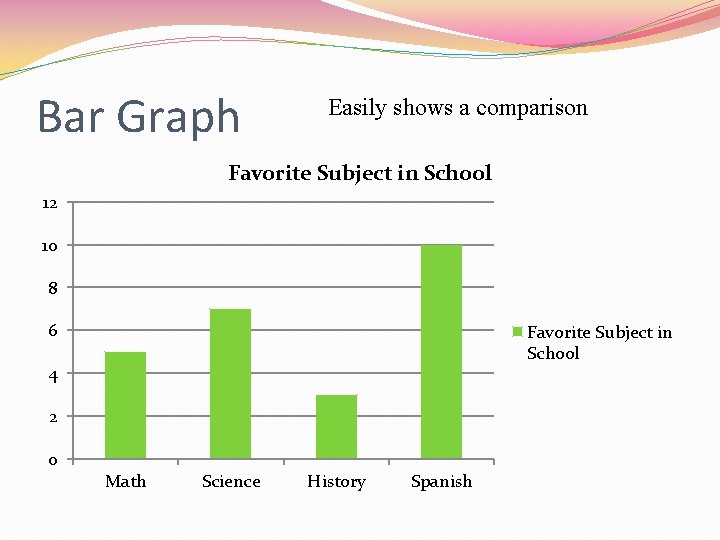
Bar Graph Easily shows a comparison Favorite Subject in School 12 10 8 6 Favorite Subject in School 4 2 0 Math Science History Spanish

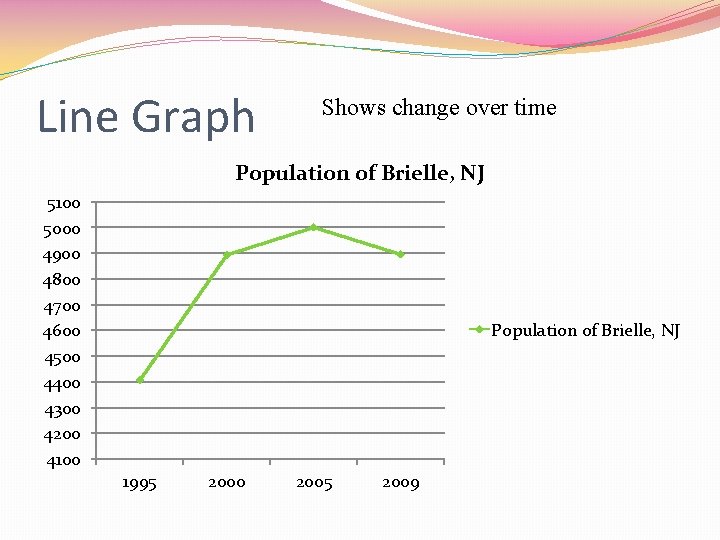
Line Graph Shows change over time Population of Brielle, NJ 5100 5000 4900 4800 4700 4600 4500 4400 4300 4200 4100 Population of Brielle, NJ 1995 2000 2005 2009

The Metric System is Easy! �Can you multiply numbers by 10? �Can you divide numbers by 10? �…then you’re on your way to becoming a metric expert!

The Metric System �Universal language of measurement �SI base units are used for consistency �Scientist all over the world use, thus making it easy to communicate. �Units can be made into derived or combined units- ex. g/ml or cm 3

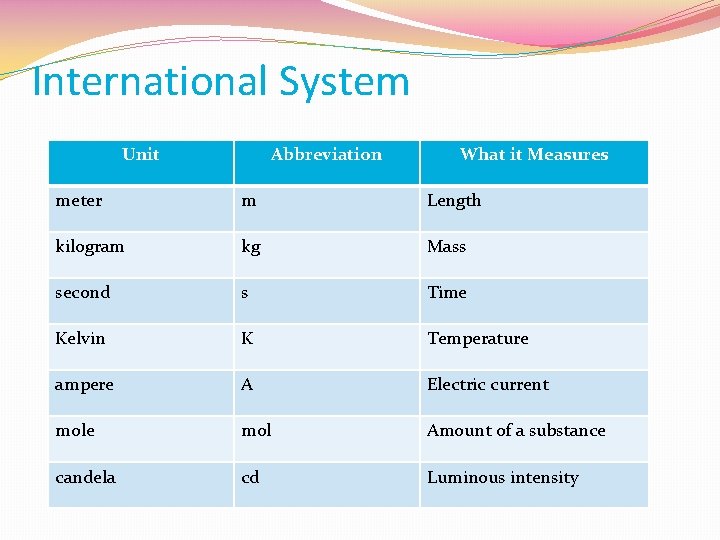
International System Unit Abbreviation What it Measures meter m Length kilogram kg Mass second s Time Kelvin K Temperature ampere A Electric current mole mol Amount of a substance candela cd Luminous intensity

Metric System �Prefixes identify large and small measurements. �easy to convert from each other. �based on a factor of 10

Metric Conversion Multiply Divide

Remember… �To convert from a larger to smaller unit : multiply �To convert from a smaller to larger unit : divide �The Latin prefixes used in the metric system literally mean the number they represent. �Example: 1 kilogram = 1000 grams A kilo is 1000 of something just like a dozen is 12 of something. � 1 kg = 1000 g also represents a conversion factor

Practice! �Guided Practice: �A roll of copper wire contains 15 m of wire. What is the length of copper wire in centimeters (cm)? �Given meters, need centimeters �How many cm in 1 m? � 100 cm = 1 m �Going larger to smaller � 15 m = 100 cm 1 m Check your work! = 15 x 100 = multiply!

Practice! �Convert the following! � 550 millimeters = ____ meters � 1. 6 kilograms = _______ grams � 4 centimeters = _______ meters �Worksheet

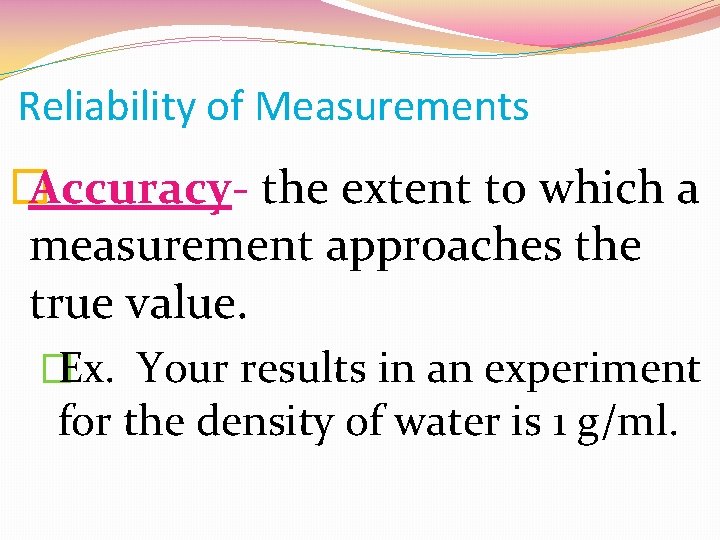
Reliability of Measurements � Accuracy- the extent to which a measurement approaches the true value. �Ex. Your results in an experiment for the density of water is 1 g/ml.

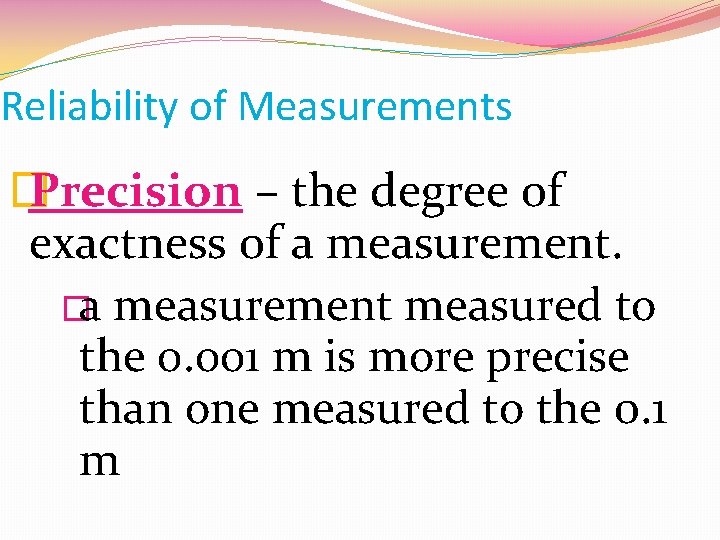
Reliability of Measurements � Precision – the degree of exactness of a measurement. �a measurement measured to the 0. 001 m is more precise than one measured to the 0. 1 m

Spelling and accuracy and precision! � Accurate: Manasquan � Inaccurate: Manesqan � Precise: Manesqan


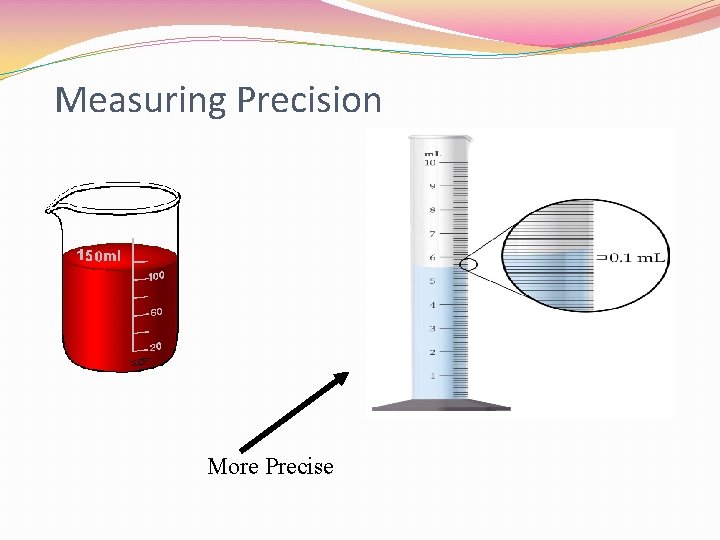
Measuring Precision More Precise

Measuring Precision �Significant Digits (figures) �Numbers that contain an actual measured value �Measures a number’s precision �Six Rules

Sig Fig Rules 1. All non-zero digits are significant. Ex. 4567 has 4 sig figs Ex. 34. 989 has 5 sig figs 2. Any zeros between two significant digits are significant Ex. 45078 has 5 sig figs Ex. 45. 0387 has 6 sig figs

Sig Fig Rules 3. All final zeros after the decimal point are significant. Ex. 2. 00 has 3 sig figs Ex. 34. 900 has 5 sig figs 4. Any zeros used solely for spacing the decimal point are NOT significant. The zeros are just place holders. Ex. 7000 has 1 sig figs Ex. 0. 00783 has 3 sig figs Ex. However 7000. has 4 sig figs

Sig Fig Rules 5. The product or quotient will be reported as having as many significant digits as the number with the least significant digits. Ex. 12. 65 x 42. 1 = 532. 565 =533 6. The sum or difference must have the same number of decimal places than the number with the least number of decimal places. Ex. 10. 13 + 20. 5 = 30. 63 = 30. 6



SCIENTIFIC NOTATION �A way of expressing really big or small numbers. �N x 10 A �N is the proper significant digit �A is the number of times you moved decimal �Positive = big, Negative = small �Only one digit before the decimal!