Chapter 1 Bonding and Molecular Structure CH 1
Chapter 1: Bonding and Molecular Structure CH 1 -2 Hybridization & Molecular Geometry • Chemical formula and Lewis Structures • Constitutional Isomers • Covalent bonds (sigma and pi) • Hybridization • Molecular geometry • Formal Charge • Dash formula, condensed formula, zig-zag formula
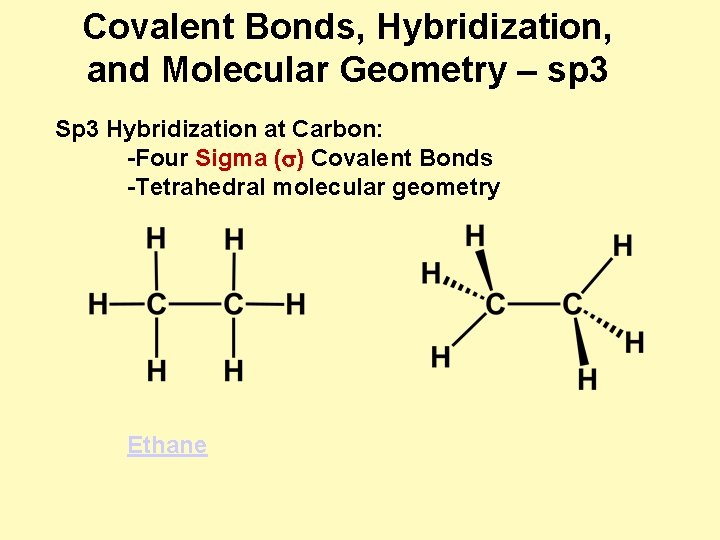
Covalent Bonds, Hybridization, and Molecular Geometry – sp 3 Sp 3 Hybridization at Carbon: -Four Sigma (s) Covalent Bonds -Tetrahedral molecular geometry Ethane
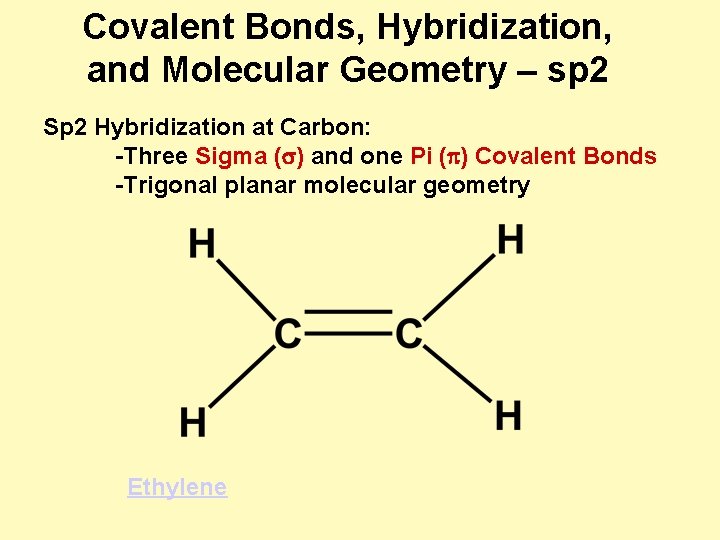
Covalent Bonds, Hybridization, and Molecular Geometry – sp 2 Sp 2 Hybridization at Carbon: -Three Sigma (s) and one Pi (p) Covalent Bonds -Trigonal planar molecular geometry Ethylene
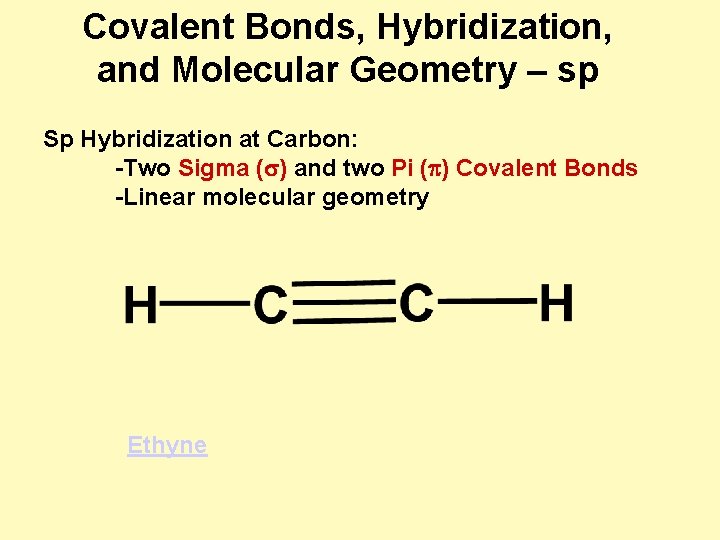
Covalent Bonds, Hybridization, and Molecular Geometry – sp Sp Hybridization at Carbon: -Two Sigma (s) and two Pi (p) Covalent Bonds -Linear molecular geometry Ethyne
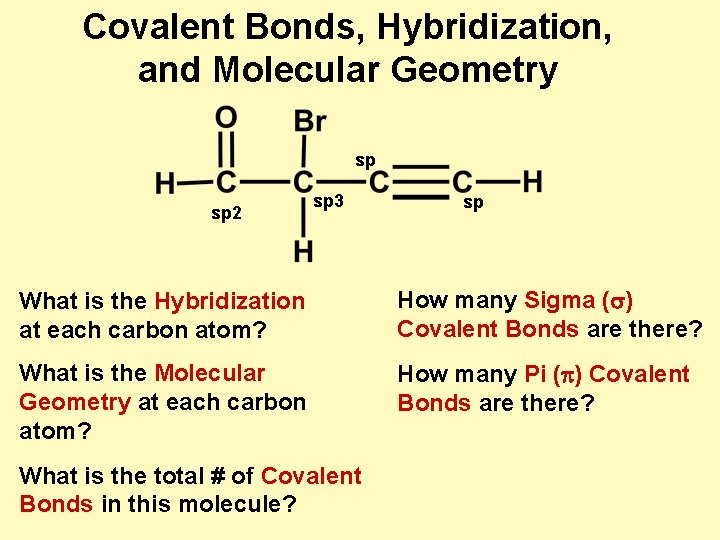
Covalent Bonds, Hybridization, and Molecular Geometry sp sp 2 sp 3 sp What is the Hybridization at each carbon atom? How many Sigma (s) Covalent Bonds are there? What is the Molecular Geometry at each carbon atom? How many Pi (p) Covalent Bonds are there? What is the total # of Covalent Bonds in this molecule?
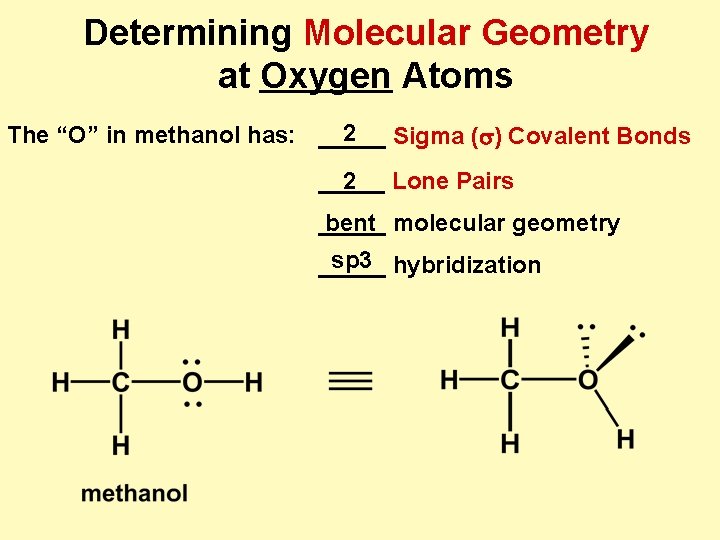
Determining Molecular Geometry at Oxygen Atoms 2 The “O” in methanol has: _____ Sigma (s) Covalent Bonds _____ 2 Lone Pairs _____ bent molecular geometry sp 3 hybridization _____
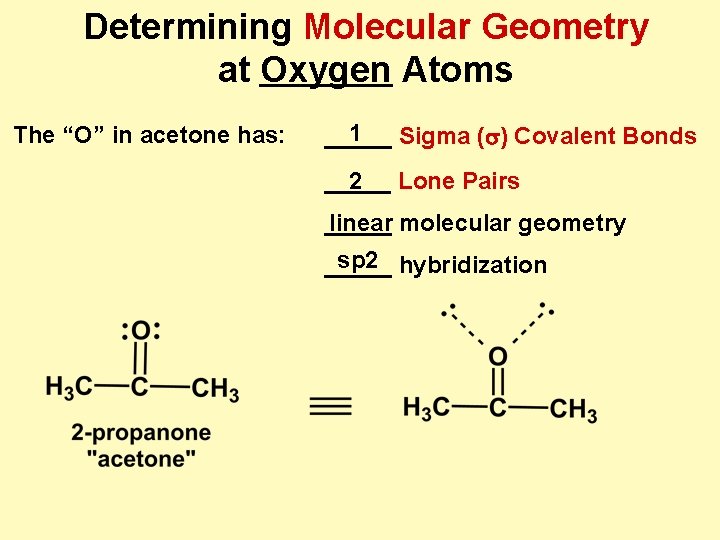
Determining Molecular Geometry at Oxygen Atoms The “O” in acetone has: 1 _____ Sigma (s) Covalent Bonds _____ 2 Lone Pairs _____ linear molecular geometry sp 2 hybridization _____
- Slides: 7