Chapter 1 Basic Concepts Thermodynamic System Properties of

第一章 基本概念 Chapter 1. Basic Concepts Ø 热力系统(Thermodynamic System) Ø 系统的状态参数(Properties of A System) Ø 状态及平衡状态(State and Equilibrium) Ø 过程与循环(Process and Cycles)
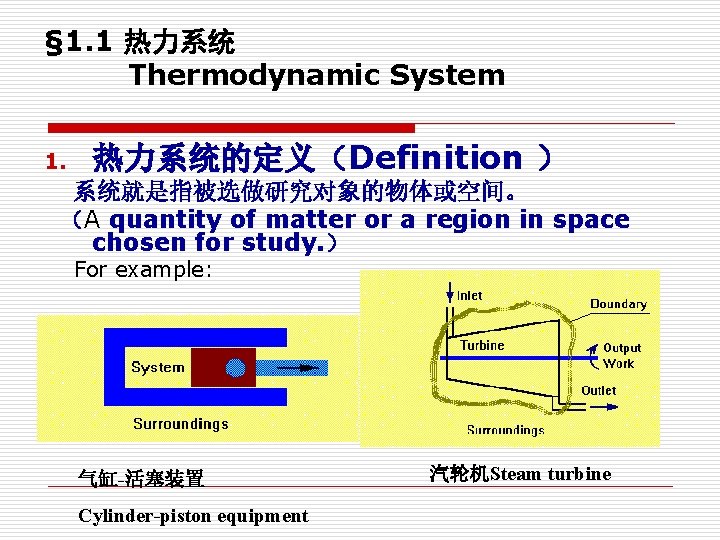
§ 1. 1 热力系统 Thermodynamic System 1. 热力系统的定义(Definition ) 系统就是指被选做研究对象的物体或空间。 (A quantity of matter or a region in space chosen for study. ) For example: 气缸-活塞装置 Cylinder-piston equipment 汽轮机Steam turbine

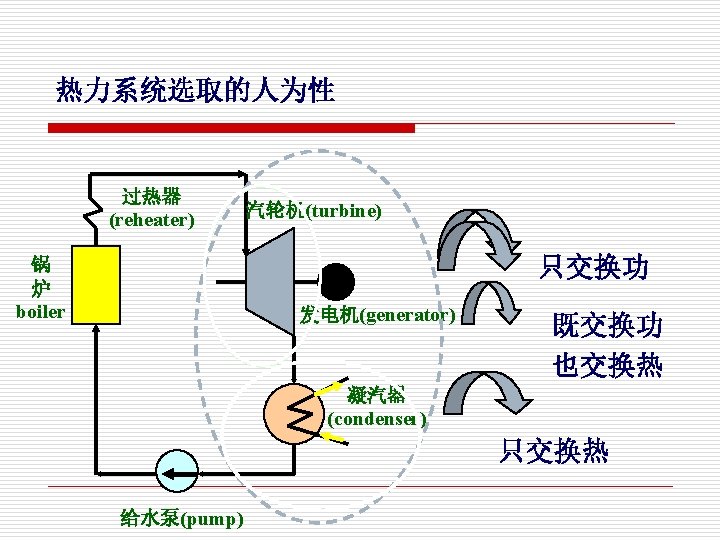
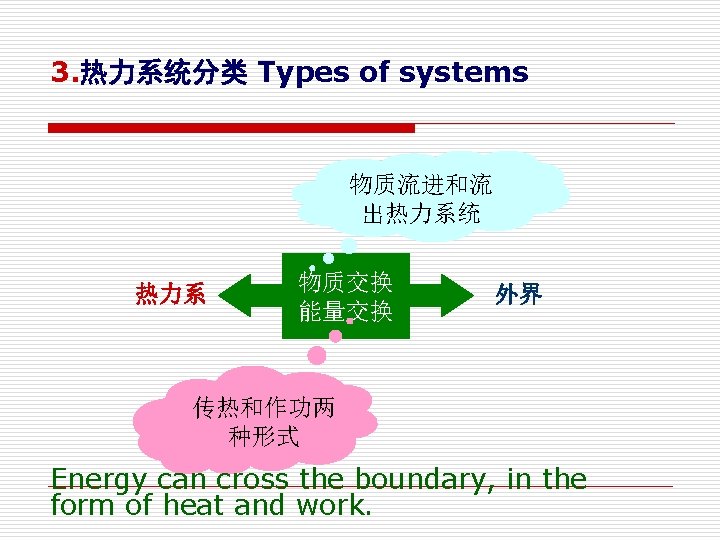
2. 系统,外界和边界 System, Surroundings and boundary (1) 外界(Surroundings) The mass or region outside the system (2)边界(Boundary) The real or imaginary surface that separates the system from its surroundings. (3) 系统与外界之间的质量及能量交换都必须穿越边界 The mass or energy exchange between system and its surroundings must cross the boundaries.

(4) Characteristics of Boundary § 边界是人为选定的 Boundaries are selected subjectively. 边界可以是固定的,也可以是可移动的 Boundaries can be fixed or movable. n 边界可以是真实的,也可以是假想的 Boundaries can be real or imaginary. n
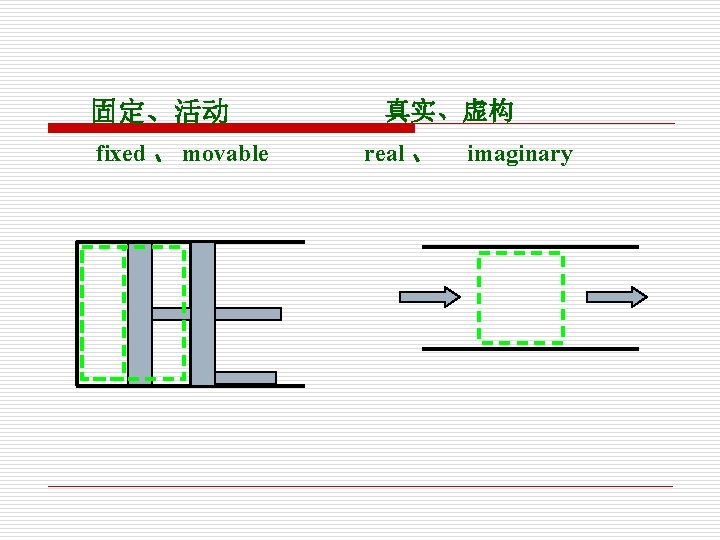
固定、活动 fixed 、 movable 真实、虚构 real 、 imaginary


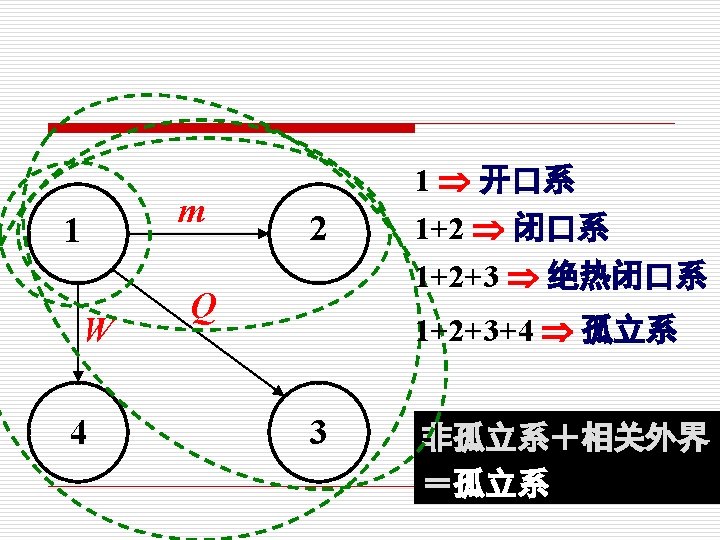
A. 闭口系统和开口系统 Closed system and Open system 闭口系与外界无物质交换 A Closed system (a control mass 控制质量) consists of a fixed amount of mass, and no mass can cross its boundary. That is, no mass enters or leave a closed system. such as, Piston-cylinder device (汽缸-活塞装置)

开口系与外界有物质交换 An Open system (or a control volume 控制 体积)is a properly selected region in space. Both mass and energy can cross the boundary of a control volume. such as, A Water heater, a turbine and a compressor, etc

B. 绝热系统和孤立系统 Adiabatic system and Isolated system 绝热系统与外界之间无热量交换 Adiabatic system is that no heat cross the boundary or heat is negligible compared with work cross the boundary 孤立系统与外界之间无任何物质和能量交换 Isolated system is a special case that no mass and energy cross the boundary.
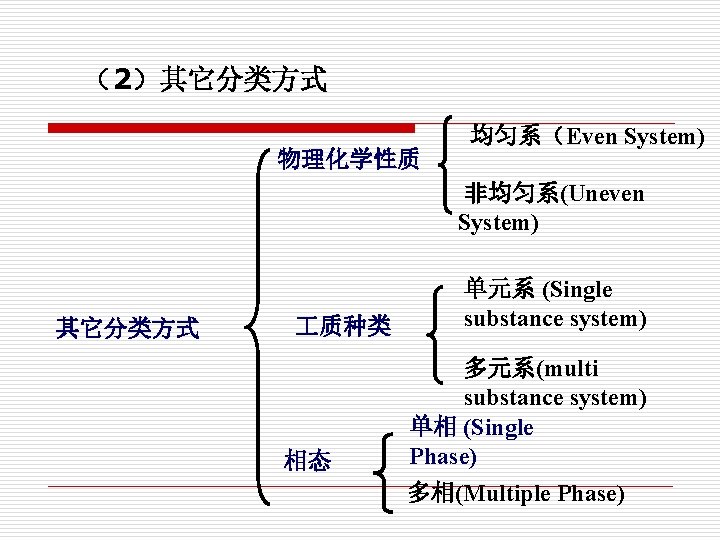
(2)其它分类方式 物理化学性质 均匀系(Even System) 非均匀系(Uneven System) 其它分类方式 质种类 相态 单元系 (Single substance system) 多元系(multi substance system) 单相 (Single Phase) 多相(Multiple Phase)
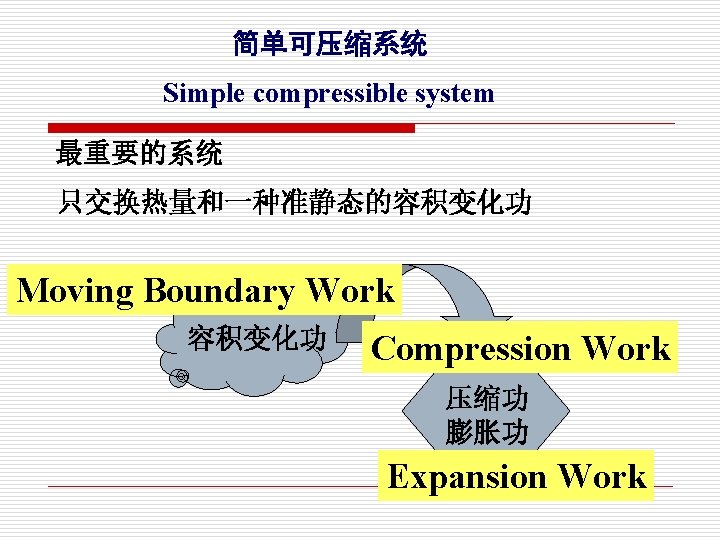
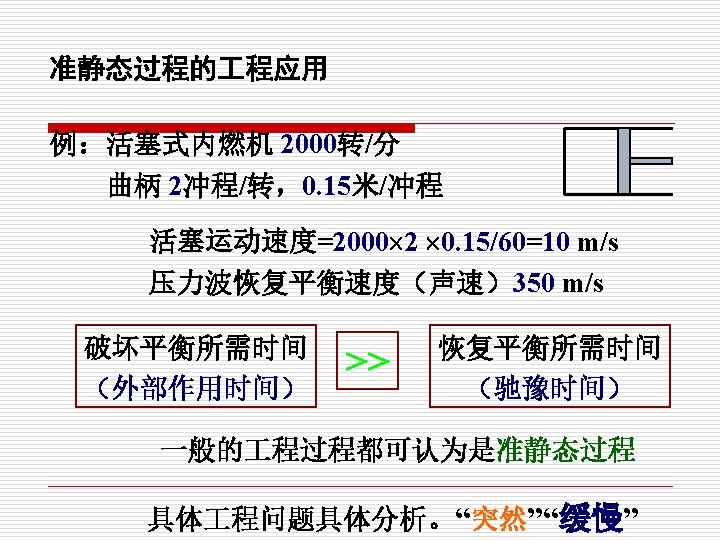
简单可压缩系统 Simple compressible system 最重要的系统 只交换热量和一种准静态的容积变化功 Moving Boundary Work 容积变化功 Compression Work 压缩功 膨胀功 Expansion Work
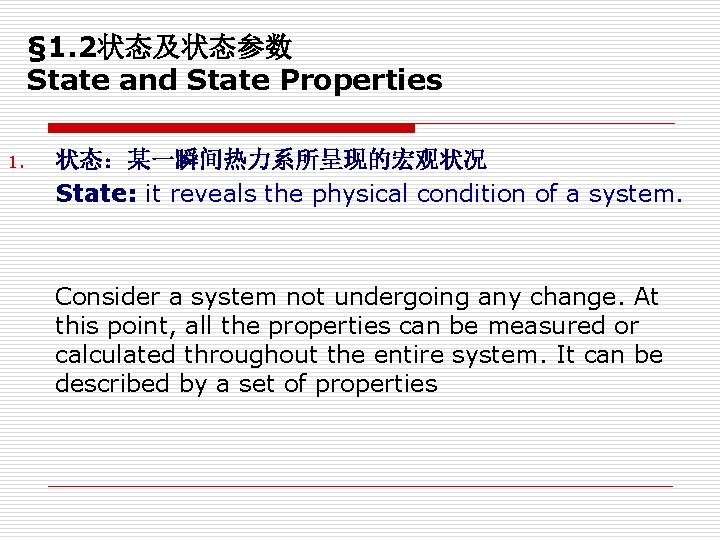
§ 1. 2状态及状态参数 State and State Properties 1. 状态:某一瞬间热力系所呈现的宏观状况 State: it reveals the physical condition of a system. Consider a system not undergoing any change. At this point, all the properties can be measured or calculated throughout the entire system. It can be described by a set of properties

2. Property (状态参数) (1)状态参数:描述系统宏观物理状况的物理量, 简称参数 Properties are used to depict any characteristic of a system. such as Pressure P (压力),temperature T(温度), volume V(体积),mass m(质量), internal energy U(内能),enthalpy(焓), entropy(熵), viscosity(粘度), thermal conductivity(导热系数)。
(2)状态参数的特征 Characteristics of State Properties A. 状态确定,则状态参数也确定,反之亦然 Properties of a state are determined by the state. If the state is specified, its properties are fixed, or vise versa. B. 状态参数的积分特征:状态参数的变化量与路径无关,只 与初终态有关 The magnitude of the change in property is independent of the path (route), but just depend on the initial and final states.
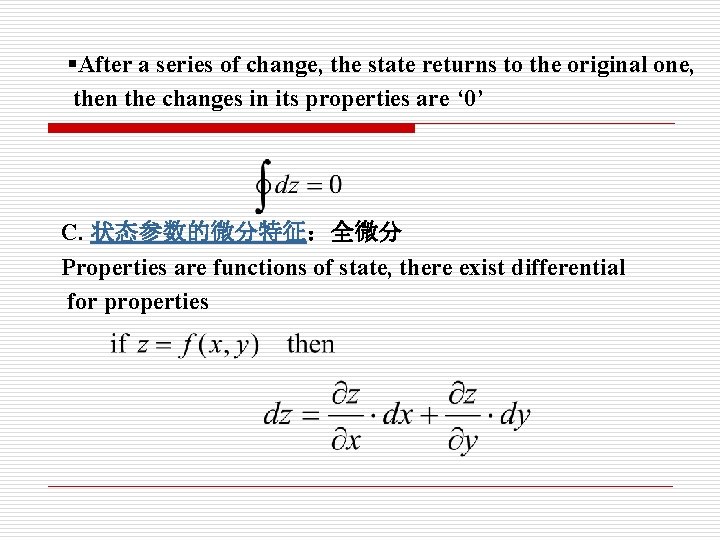
§After a series of change, the state returns to the original one, then the changes in its properties are ‘ 0’ C. 状态参数的微分特征:全微分 Properties are functions of state, there exist differential for properties
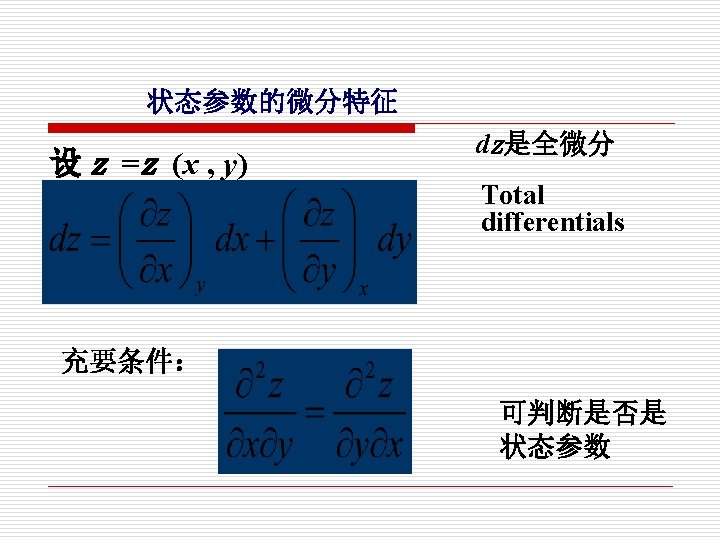

3. 基本状态参数 Basic State Properties 压力 p、温度 T、比容 v (容易测量) (1)密度和比容 Density and Specific Volume 密度指单位体积所含物质的多少。 Density is defined as mass per unit volume.

比体积 单位质量的物质所占有的体积,用v表示。 Specific volume is the reciprocal of density and is defined as volume per unit mass. 比体积是表示物质内部分子疏密程度的状态参数。

(2) 压力 Pressure 定义 Definition: 垂直作用于单位面积上的力 It is defined as the force exerted by a fluid vertically on a surface of unit area. ① 绝对压力 (for solid is stress: person stand on foot) ②压力的单位 Unit of pressure It has the unit of Newtons per square meter (N/m 2) 1 Pa=1 N/m 2 1 k. Pa= 103 Pa 1 MPa= 106 Pa SI单位制

其他单位 Other units 1 bar =105 Pa=100 k. Pa =0. 1 MPa 标准大气压 Standard atmosphere 1 atm= 101325 Pa=101. 325 k. Pa 程大气压 Engineer atmosphere 1 at=1 kgf/cm 2 =9. 807 N/cm 2 =9. 807*104 Pa 液柱高度 Height of liquid column 1 atm=760 mm Hg 1 at =10 m H 2 O 1 mm. Hg=1ρgh =133. 3 Pa

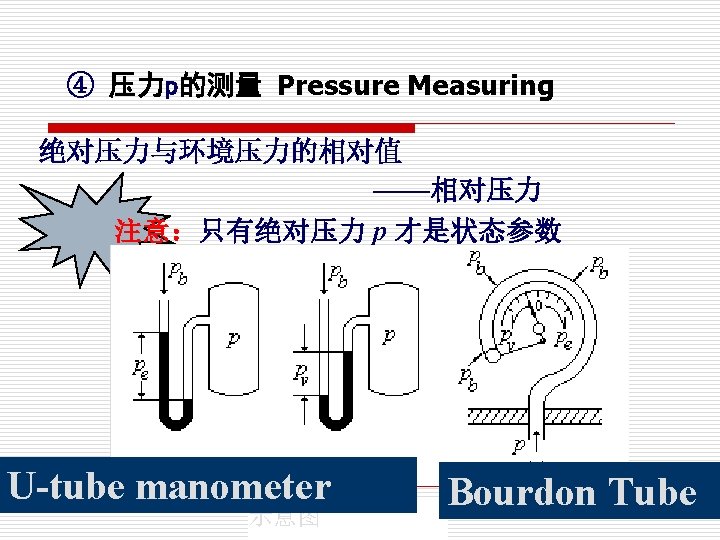
③ 绝对压力和相对压力 Absolute pressure and relative pressure 绝对压力指系统中 质的真实压力。 Absolute pressure is the actual pressure at a given position P. 相对压力反映绝对压力与当地大气压力的差值。 Relative pressure indicates the difference between the absolute pressure and the local atmospheric pressure.

④ 表压与真空度 Gage Pressure and Vacuum Pressure o 表压力:当高于大气压时,压力计显示的绝对压力 超出大气压力的部分。 Gage pressure(表压力)is denoted as Pg 表压力=绝对压力-大气压力 真空度表示绝对压力低于大气压力的量值 Vacuum pressure (真空度):Pressures below atmosphere pressure. It is the pressure difference between atmospheric and system pressure when system pressure is lower than atmospheric And it is denoted as PVAC or H. 真空度=大气压力-绝对压力
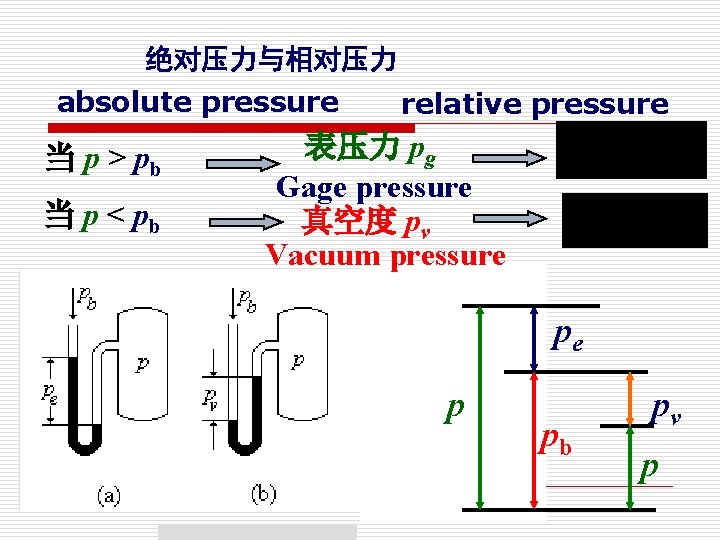
绝对压力与相对压力 absolute pressure 当 p > pb 当 p < pb relative pressure 表压力 pg Gage pressure 真空度 pv Vacuum pressure pe p pb pv p


Variation of pressure with Depths § Pressure is a scalar quantity. (压力是一个标量) At any point in a fluid, Pressure is the same in all directions. n Pressure in a fluid increases linearly with depth. (液体中的压力随液体的深度线性增加) n Pressure is the same at all points on a horizontal plane in a given continuous fluid at rest. (帕斯卡定律).

大气压力Atmospheric pressure 大气压随时间、地点变化 物理大气压 1 atm = 760 mm. Hg 1 mm. Hg = ρgh = 133. 322 Pa
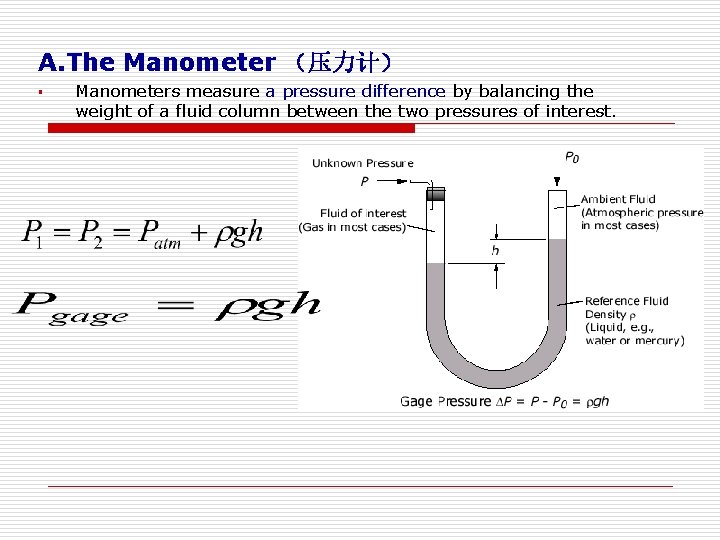
A. The Manometer (压力计) § Manometers measure a pressure difference by balancing the weight of a fluid column between the two pressures of interest.
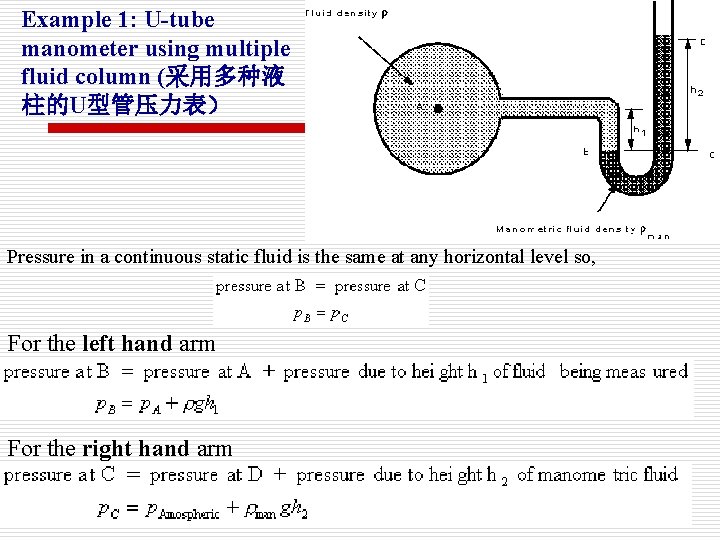
Example 1: U-tube manometer using multiple fluid column (采用多种液 柱的U型管压力表) Pressure in a continuous static fluid is the same at any horizontal level so, For the left hand arm For the right hand arm
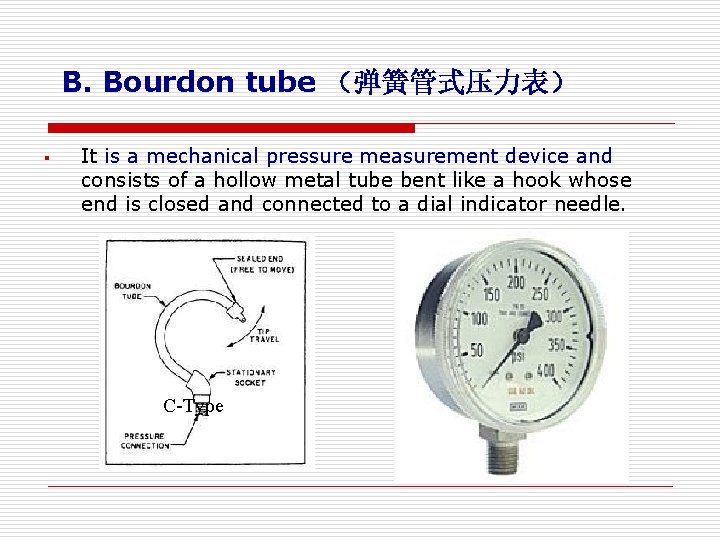
B. Bourdon tube (弹簧管式压力表) § It is a mechanical pressure measurement device and consists of a hollow metal tube bent like a hook whose end is closed and connected to a dial indicator needle. C-Type
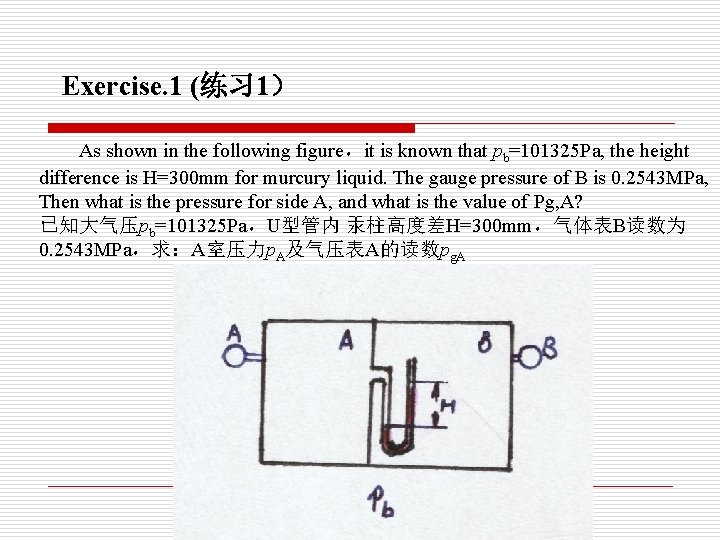
Exercise. 1 (练习 1) As shown in the following figure,it is known that pb=101325 Pa, the height difference is H=300 mm for murcury liquid. The gauge pressure of B is 0. 2543 MPa, Then what is the pressure for side A, and what is the value of Pg, A? 已知大气压pb=101325 Pa,U型管内 汞柱高度差H=300 mm,气体表B读数为 0. 2543 MPa,求:A室压力p. A及气压表A的读数pg. A
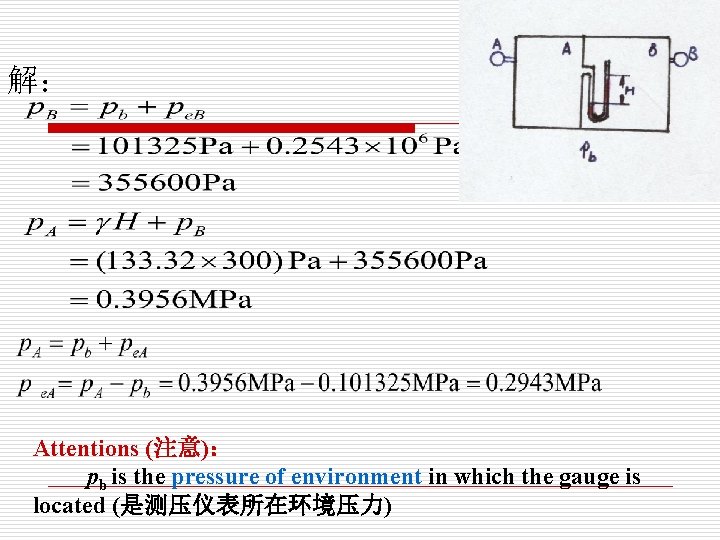
解: Attentions (注意): pb is the pressure of environment in which the gauge is located (是测压仪表所在环境压力)
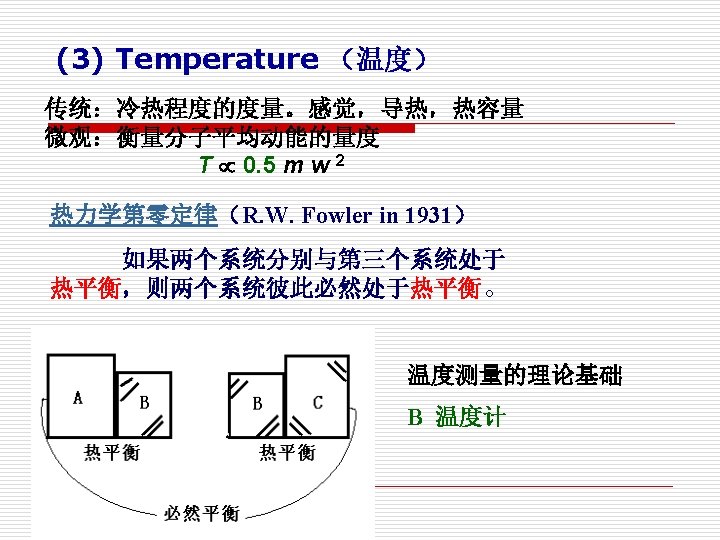
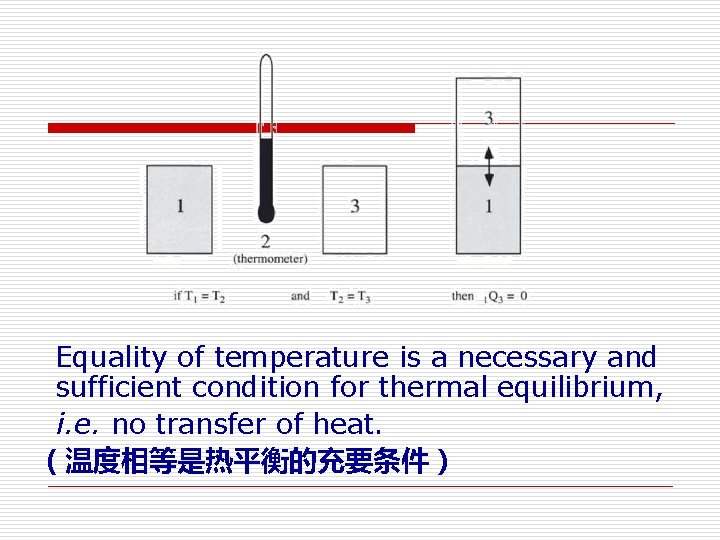
Equality of temperature is a necessary and sufficient condition for thermal equilibrium, i. e. no transfer of heat. (温度相等是热平衡的充要条件)
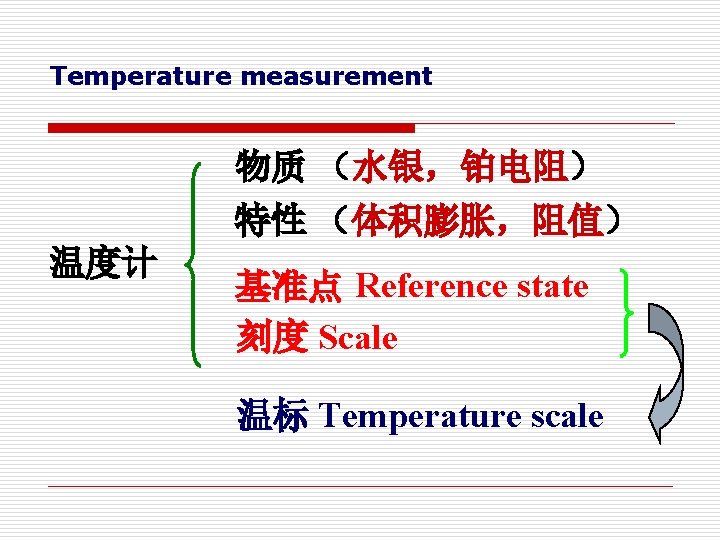
Temperature measurement 物质 (水银,铂电阻) 特性 (体积膨胀,阻值) 温度计 基准点 Reference state 刻度 Scale 温标 Temperature scale
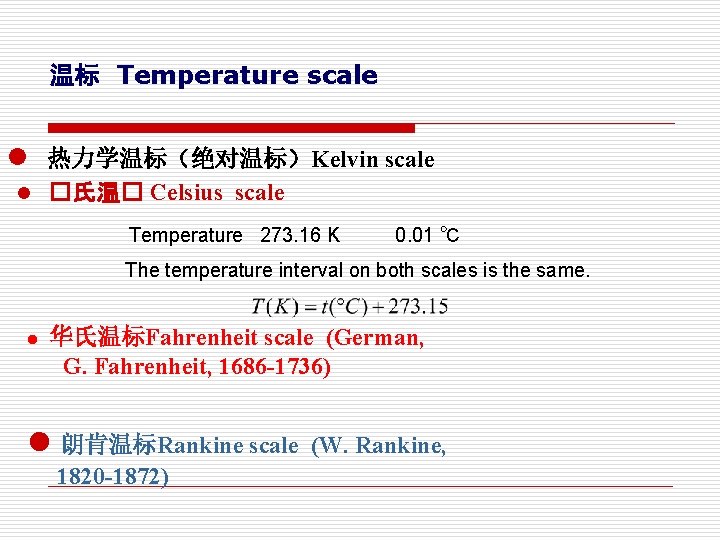
温标 Temperature scale l 热力学温标(绝对温标)Kelvin scale l �氏温� Celsius scale Temperature 273. 16 K 0. 01 ℃ The temperature interval on both scales is the same. l 华氏温标Fahrenheit scale (German, G. Fahrenheit, 1686 -1736) l 朗肯温标Rankine scale (W. Rankine, 1820 -1872)
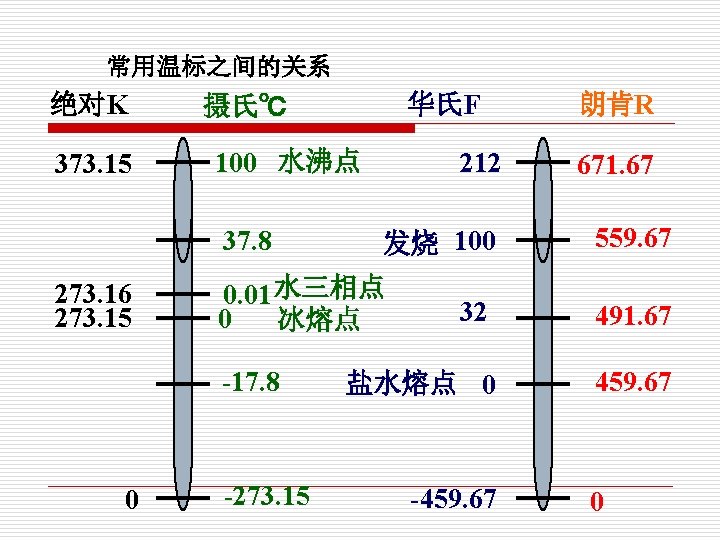

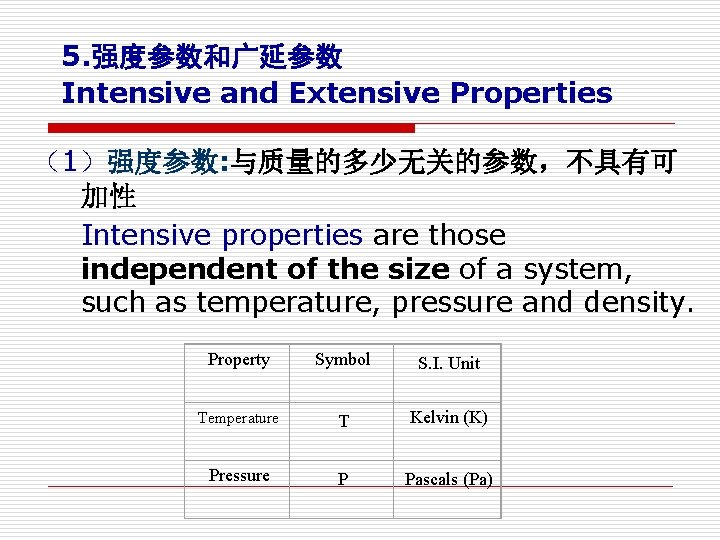
5. 强度参数和广延参数 Intensive and Extensive Properties (1)强度参数: 与质量的多少无关的参数,不具有可 加性 Intensive properties are those independent of the size of a system, such as temperature, pressure and density. Property Symbol S. I. Unit Temperature T Kelvin (K) Pressure P Pascals (Pa)

(2) 广延参数: 与物质的量有关的参数,具有可加性) Extensive properties are those whose values depend on the size or extent of the system 如 质量m、容积 V、内能 U、焓 H、 such as mass, volume, internal energy and enthalpy
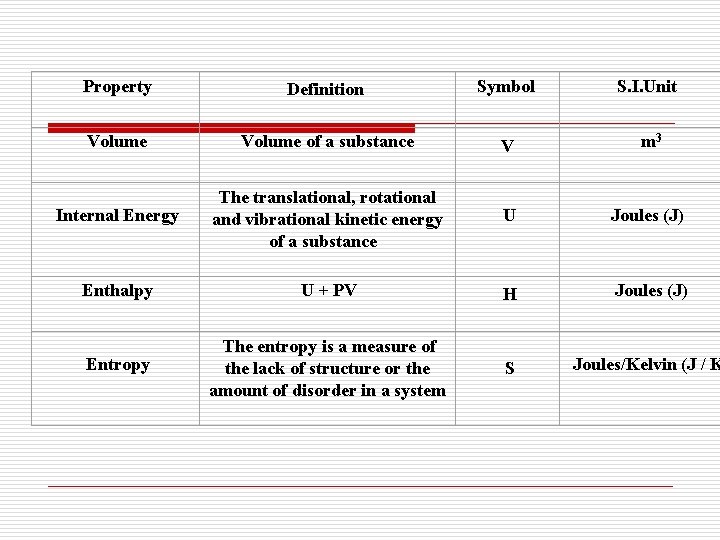
Property Definition Symbol S. I. Unit Volume of a substance V m 3 Internal Energy The translational, rotational and vibrational kinetic energy of a substance U Joules (J) Enthalpy U + PV H Joules (J) S Joules/Kelvin (J / K Entropy The entropy is a measure of the lack of structure or the amount of disorder in a system

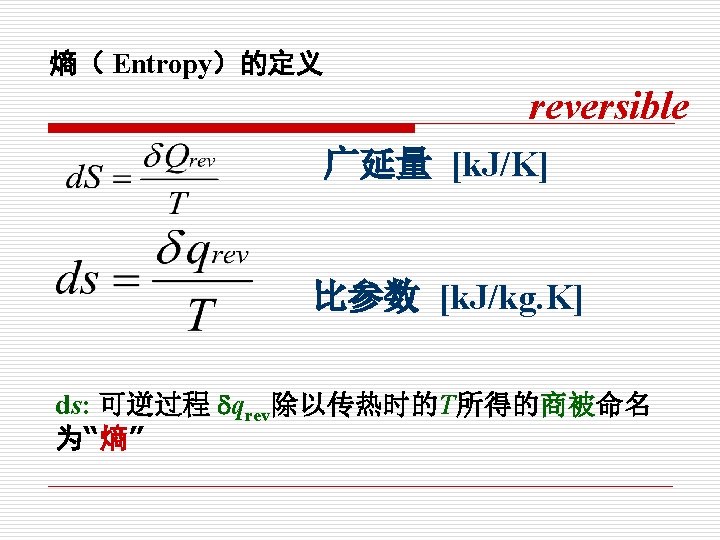
比参数: l 比参数是单位质量的广延参数 Specific properties is extensive properties per unit mass. 比容 比内能 比焓 比熵
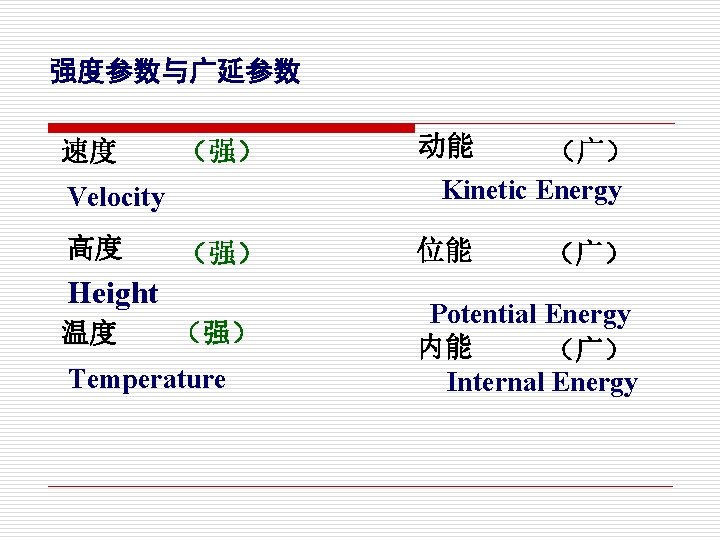
强度参数与广延参数 速度 (强) 动能 (强) 位能 Velocity 高度 Height 温度 (强) Temperature (广) Kinetic Energy (广) Potential Energy 内能 (广) Internal Energy

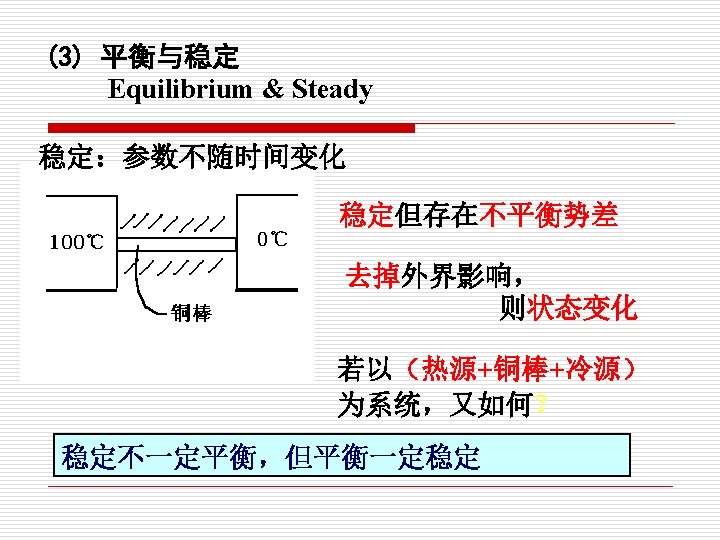
§ 1. 3 平衡状态和状态公理 Equilibrium State and State Postulate 1. 平衡状态(Equilibrium State) (1) 定义 Definition 所谓平衡状态就是指在没有外界影响的情况下,系统的 状态不随时间而发生变化。 A system in equilibrium experiences no changes with time when it is isolated from its surroundings.
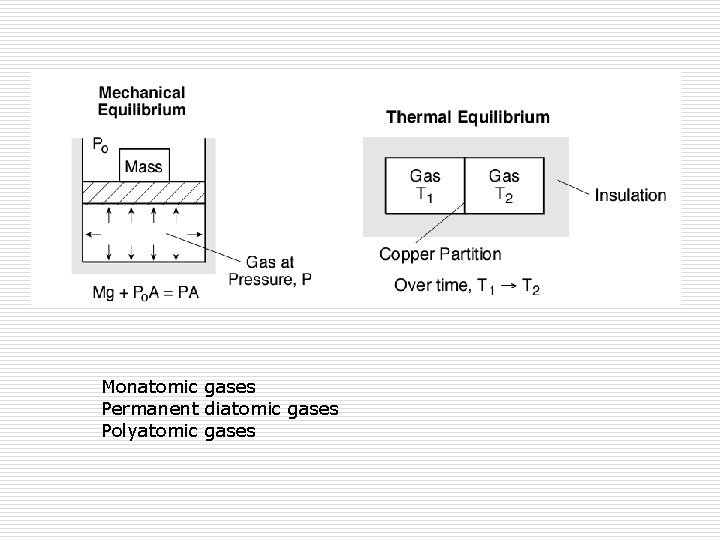
Monatomic gases Permanent diatomic gases Polyatomic gases

(2)如何实现热力学平衡 How to fulfill thermodynamic equilibrium? A. 平衡状态下,系统内外不存在不平衡势差(推动力 In an equilibrium state there are no unbalance potentials (or driving forces) B. A system is not in thermodynamic equilibrium unless the condition of all the relevant types of equilibrium are satisfied.
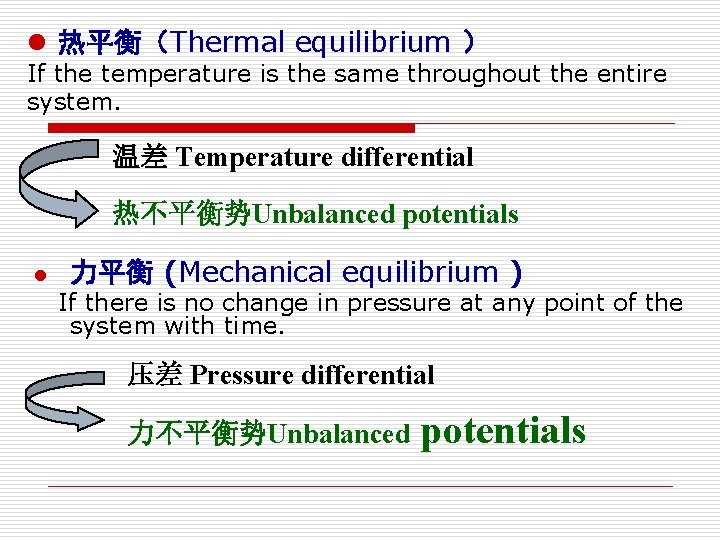
l 热平衡(Thermal equilibrium ) If the temperature is the same throughout the entire system. 温差 Temperature differential 热不平衡势Unbalanced potentials l 力平衡 (Mechanical equilibrium ) If there is no change in pressure at any point of the system with time. 压差 Pressure differential 力不平衡势Unbalanced potentials
Monatomic gases Permanent diatomic gases Polyatomic gases

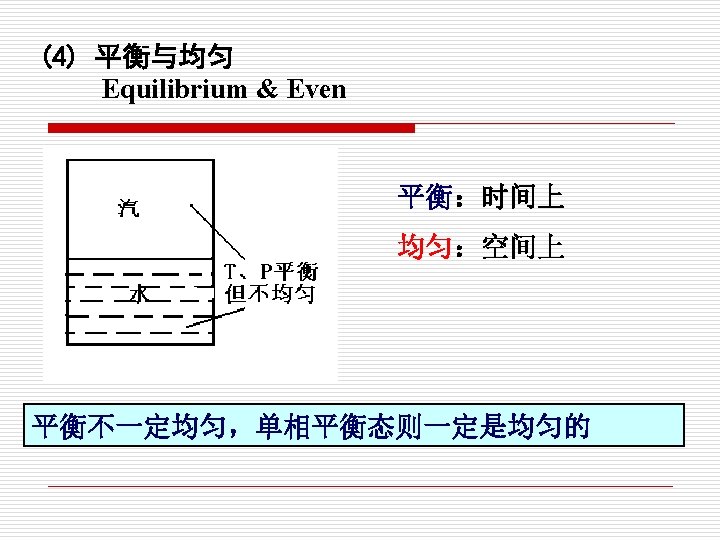
相平衡(Phase equilibrium) If a system involves two phases, when the mass of each phase reaches an equilibrium level and stays there , it is in phase equilibrium. § 化学平衡 (Chemical equilibrium ) If no chemical reaction occur, that is, the chemical composition does not change with time, a system is in chemical §


2. 状态公理 The State Postulate 系统的平衡状态可以用确定的参数来描述 The equilibrium state of a system can be described by a set of properties. However, specify a certain number of properties is sufficient to fix a state. (1) 想确切描述某个热力系,是 否需要所有状态参数?
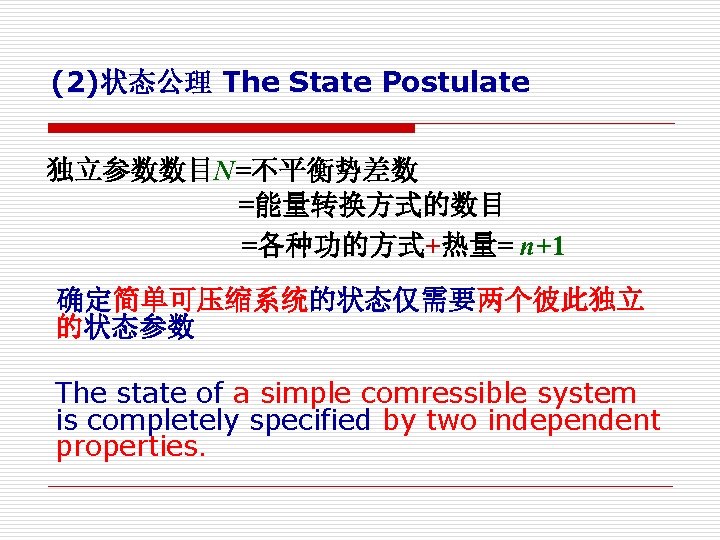
(2)状态公理 The State Postulate 独立参数数目N=不平衡势差数 =能量转换方式的数目 =各种功的方式+热量= n+1 确定简单可压缩系统的状态仅需要两个彼此独立 的状态参数 The state of a simple comressible system is completely specified by two independent properties.

§ § 简单可压缩系统 A simple compressible system is a system in the absence of electrical magnetic, gravitational motion and surface tension effects. Two independent properties: If one property can be varied while the other one is held constant. (eg. P and T are independent properties for single phase systems, but are dependent properties for multiphase systems. ) 绝热简单可压缩系统 N = ?

3. 状态方程式 Equation of State 各状态参数不是互相独立的,只要知道二个状态 参数就可以确定系统的状态。 Not all properties are independent of each other, if two independent properties are known, then other properties of the same state can be determined. l 状态方程 基本状态参数(p, v, T)之间的关系 l The relationship between properties is called Equation of State (E. O. S)
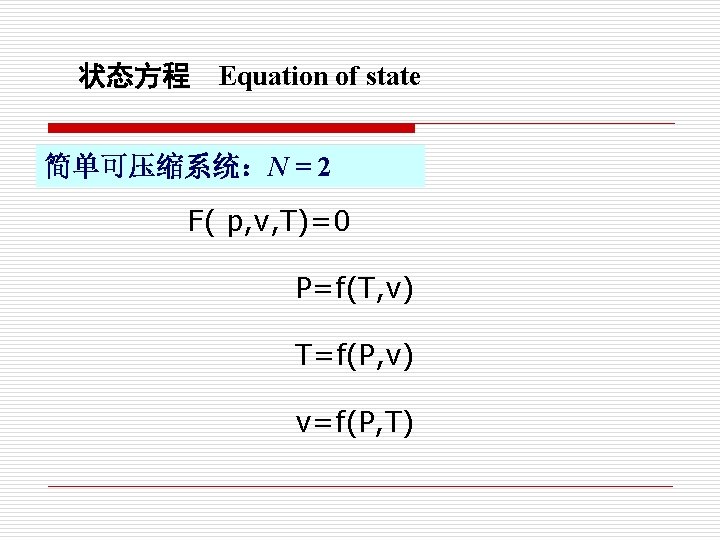
状态方程 Equation of state 简单可压缩系统:N = 2 F( p, v, T)=0 P=f(T, v) T=f(P, v) v=f(P, T)
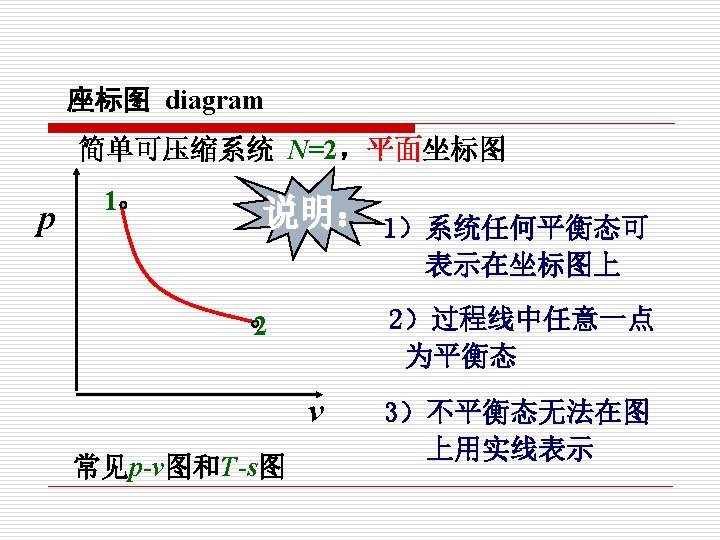
1. 准静态过程 Quasi-static or Quasi-equilibrium process (1)过程和路径(Process and path) 热力过程: 质从一个平衡状态过渡到另一个平衡 状态所经历的全部状态的总和。 Any change that a system undergoes from one equilibrium state to another is called a process. 路径: The series of states through which a system passes during a process is called the path of the process.
(2)准静态过程 Quasi-static or Quasi-equilibrium process
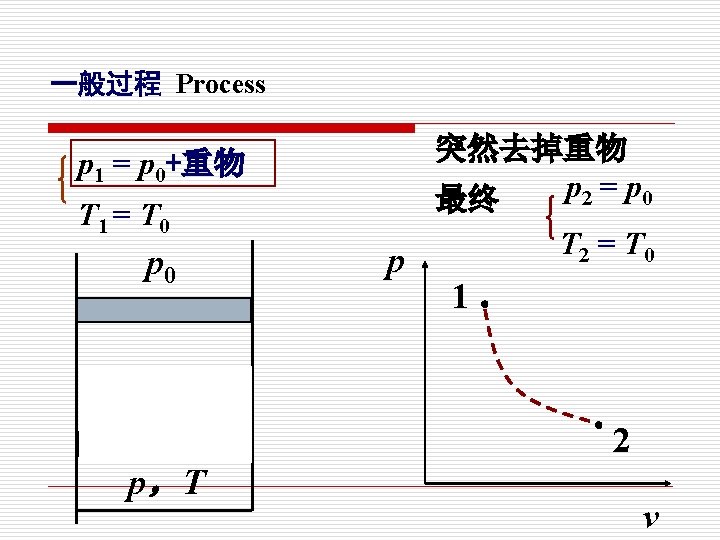
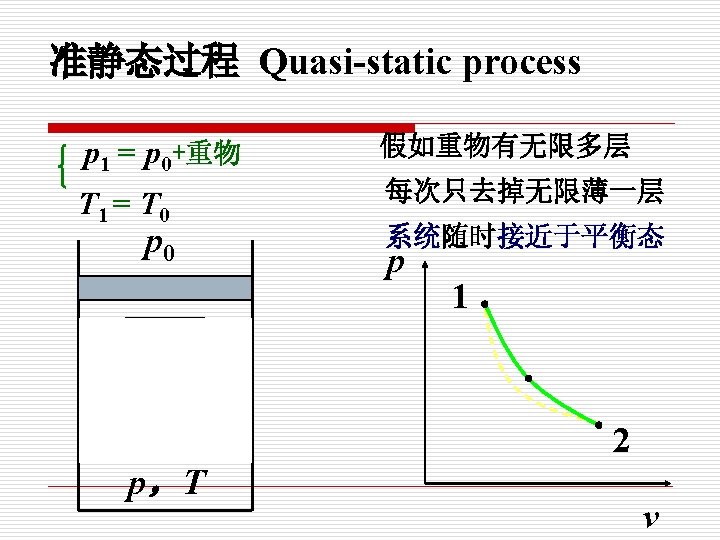

在无限小势差推动下的由连续平衡态组成的过程, 就是准静态过程。 When a process proceeds in such a manner that the system remains infinitesimally close to an equilibrium state at all times, it is called a quasistatic or quasi-equilibrium process.


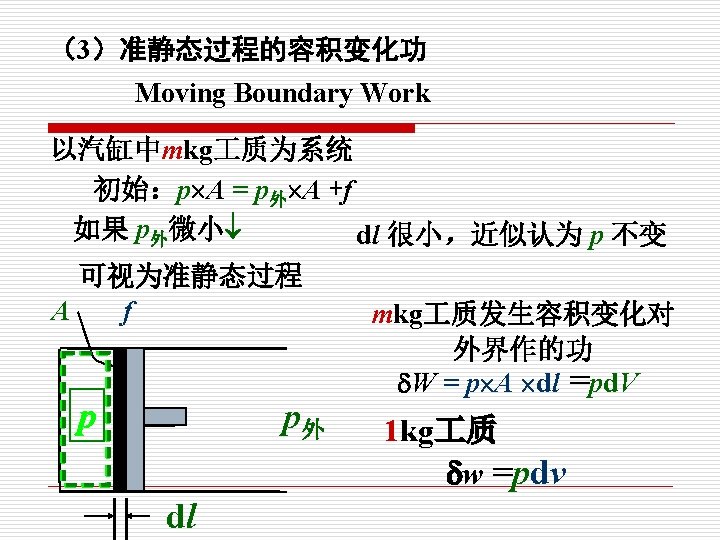
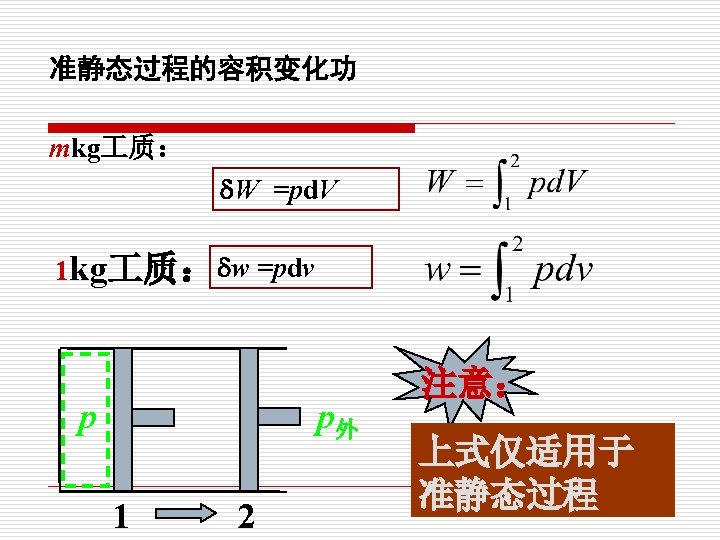
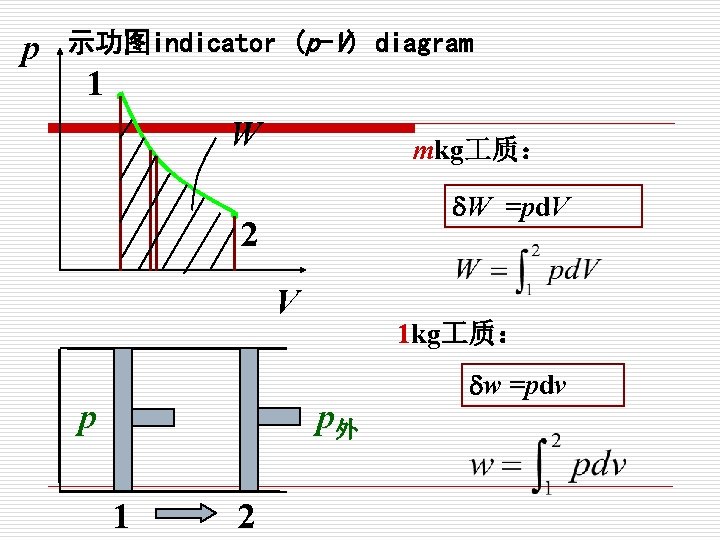
p 示功图indicator (p-V) diagram 1. W mkg 质: . W =pd. V 2 V p 1 kg 质: p外 1 2 w =pdv
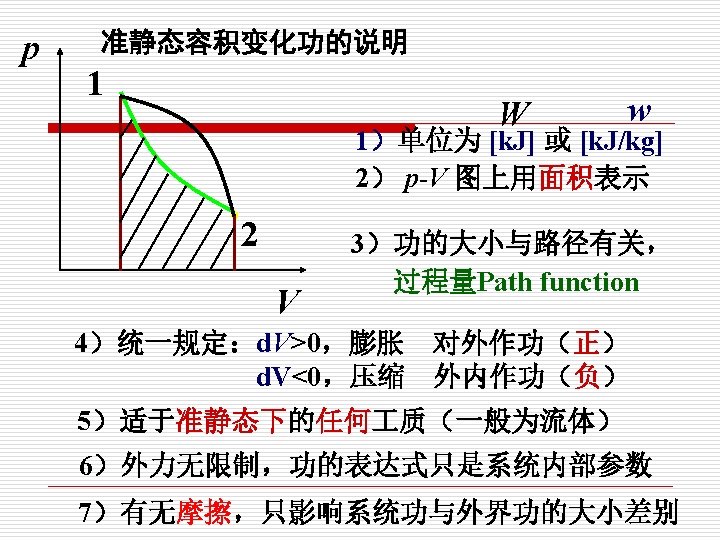
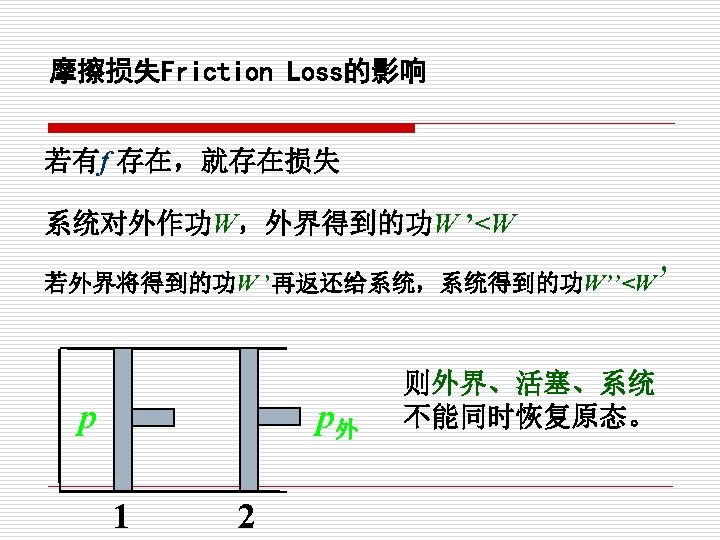
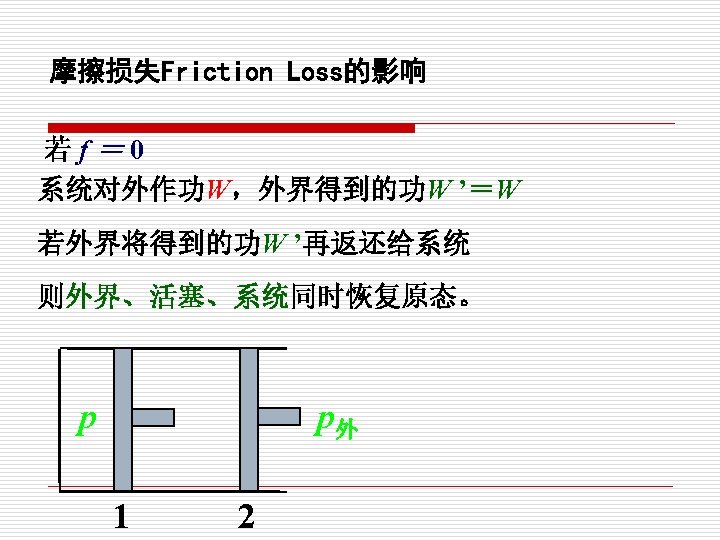

2. Reversible process (可逆过程) (1)定义(Definition) 系统经历某一过程后,如果能使系统与外界 同时恢复到初始状态,而不留下任何痕迹,则此过 程为可逆过程。 A process that can reversed without leaving any trace on the surroundings. That is, both the system 注意 and the surroundings are returned to their initial states at the end of the reverse process. 可逆过程只是指可能性,并不 是指必须要回到初态的过程。

§ 在可逆过程结束时,系统和外界都回复到原来的状态 Both the system and the surroundings are returned to their initial states at the end of the reverse process. § 系统与环境组合系统的净热量与净功量之和为零 The net heat and net work exchange between the system and the surroundings is zero for the combined ( original and reverse) process.
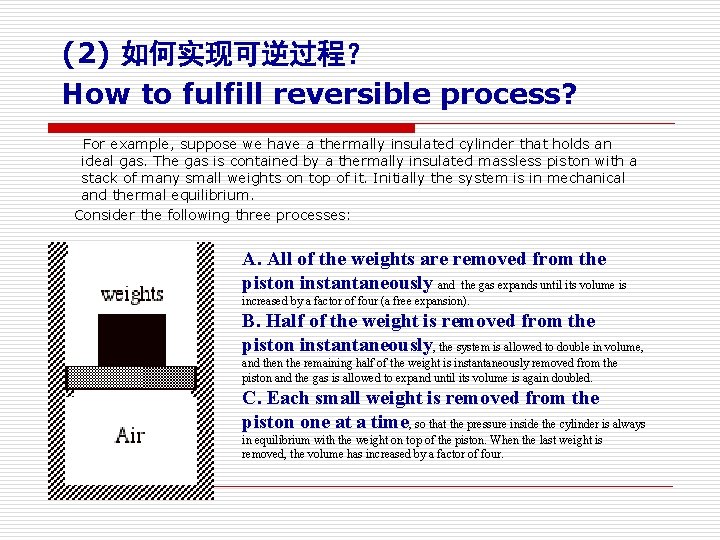
(2) 如何实现可逆过程? How to fulfill reversible process? For example, suppose we have a thermally insulated cylinder that holds an ideal gas. The gas is contained by a thermally insulated massless piston with a stack of many small weights on top of it. Initially the system is in mechanical and thermal equilibrium. Consider the following three processes: A. All of the weights are removed from the piston instantaneously and the gas expands until its volume is increased by a factor of four (a free expansion). B. Half of the weight is removed from the piston instantaneously, the system is allowed to double in volume, and then the remaining half of the weight is instantaneously removed from the piston and the gas is allowed to expand until its volume is again doubled. C. Each small weight is removed from the piston one at a time, so that the pressure inside the cylinder is always in equilibrium with the weight on top of the piston. When the last weight is removed, the volume has increased by a factor of four.
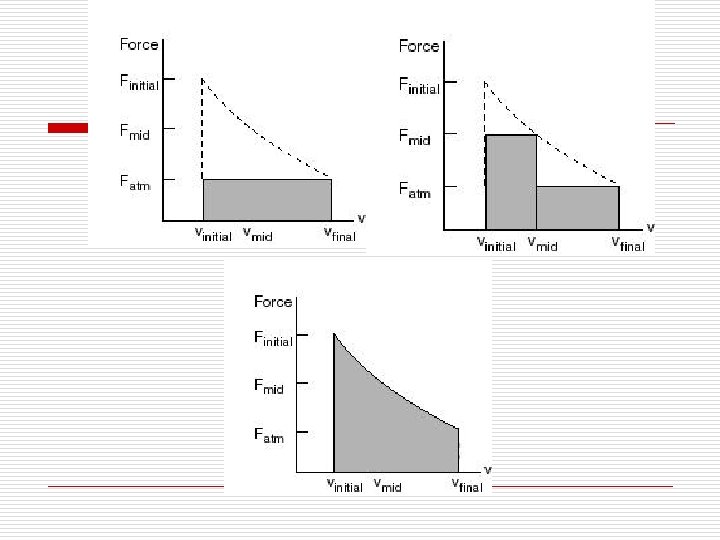

This requires that the working fluid goes through a continuous series of equilibrium states. A reversible process should satisfy the following criteria: l 质与环境之间的温差和压差必须无限小 The temperature and pressure difference between the working fluid and its surroundings should be infinitely small. l没有内部或机械摩擦损失--没有耗散效应 No internal or mechanical friction is allowed. ----no dissipative effect.
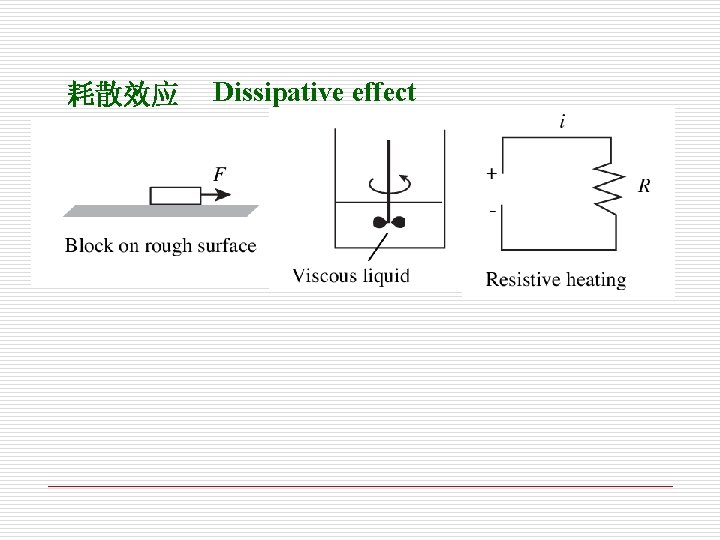
耗散效应 Dissipative effect

(3)Irreversibilities (不可逆性) The factors that cause a process to be irreversible are called irreversibilities. (导致过程不可逆的因素称为不可逆性) Such as heat transfer across a finite temperature difference(温差传热), Temperature difference is the driving force of heat transfer. However, a heat transfer process becomes less and less irreversible as the temperature difference between the two bodies approaches zero friction(摩擦), The more friction force involved, the more irreversibility the process is. (过程所涉及的摩擦阻力越大,过程的不可逆越强. ) Friction can occur between two solid bodies, and also between solid and a fluid, even between the layers of a fluid moving at different velocities.
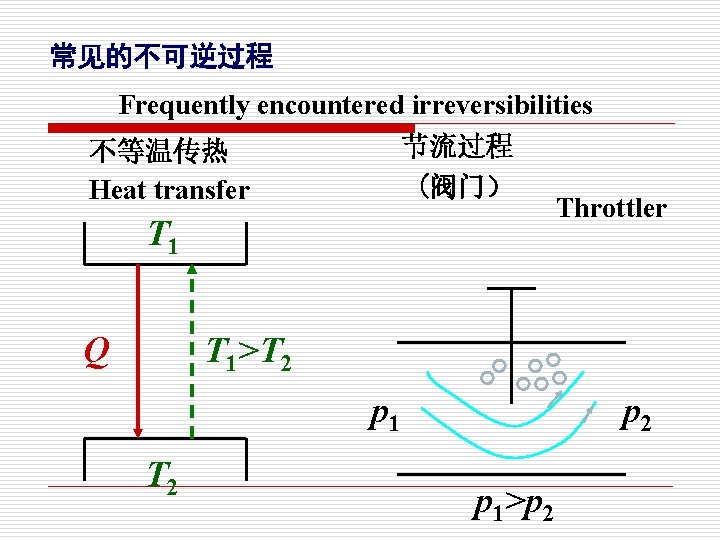
常见的不可逆过程 Frequently encountered irreversibilities 节流过程 (阀门) 不等温传热 Heat transfer T 1 Q Throttler T 1>T 2 p 1>p 2
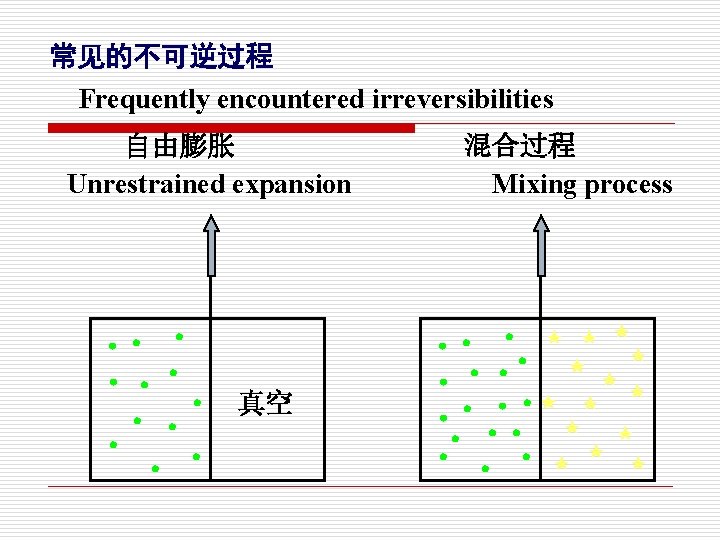
常见的不可逆过程 Frequently encountered irreversibilities 自由膨胀 Unrestrained expansion • • • 真空 混合过程 Mixing process • • • • • ★ ★ ★ ★

electric resistance(电阻) inelastic deformation of solid(固体的塑性 变形) chemical reactions(化学反应).

(4 )准静态过程与可逆过程的区别 The distinction between quasi-equilibrium and reversible process l 准静态过程是一个内部不可逆过程,而可逆过程 是完全不可逆过程。 Quasi-equilibrium process is an internally reversible process,while reversible process is a totally reversible process. l 可逆过程中不存在耗散效应。 Dissipative effects are not allowed in reversible process.

l 可逆过程必然是准静态过程,但准静态过程不一定是可逆过程 Reversible process must be quasi-equilibrium process. However,quasi-equilibrium process is not definitely reversible process. l 简单地说,可逆过程就是没有耗散效应的准静态过程 In brief, reversible process is a quasi-equilibrium process without dissipative process.


功的热力学定义Ⅱ 功是系统与外界相互作用的一种方式,在力 的推动下,通过有序运动方式传递的能量。 Work is an energy interaction between a system and its surroundings, if the energy crossing the boundary of a closed system is not heat, it must be work.
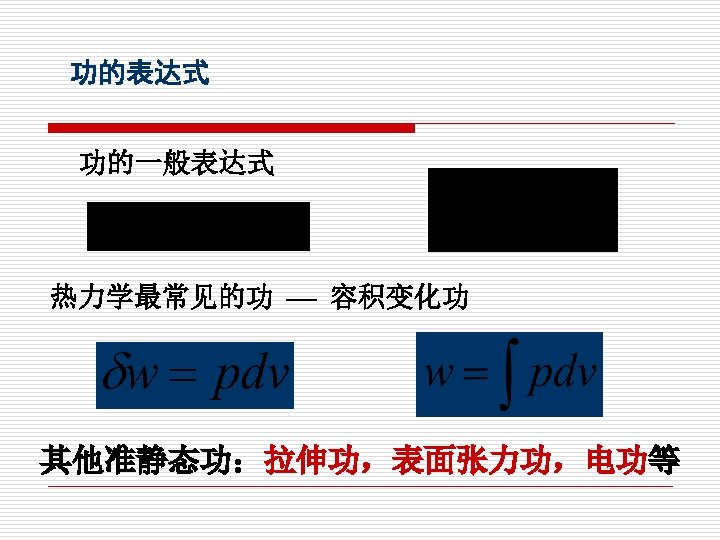

§ 1 -6 热量与熵 Heat and Entropy 1. 热量定义:热量是热力系与外界相互作用的另一 种方式,在温度的推动下,以微观无序运动方式传 递的能量。 Heat is defined as the form of energy that is transferred between two systems (or its surroundings) by virtue of a temperature difference.
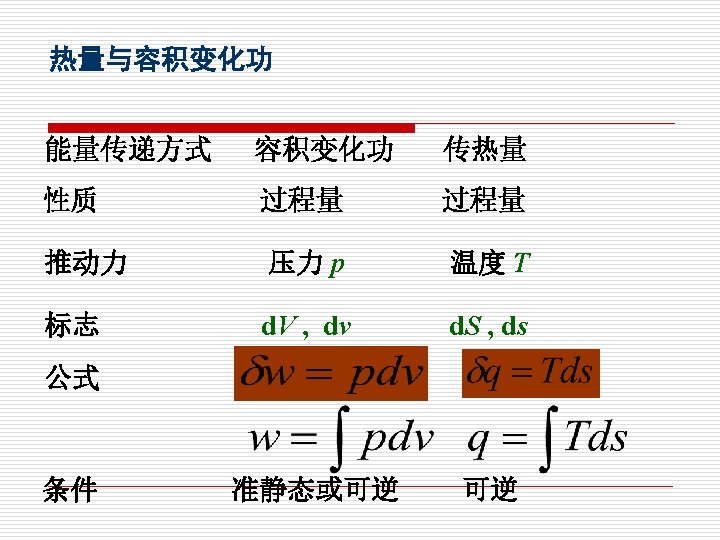

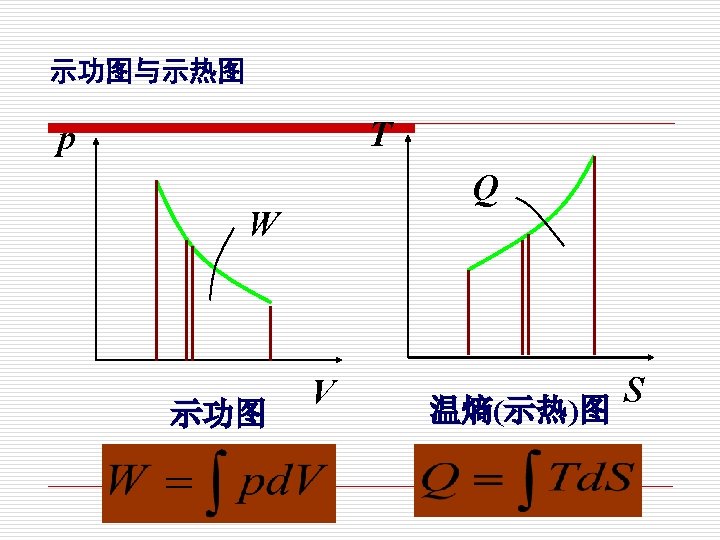
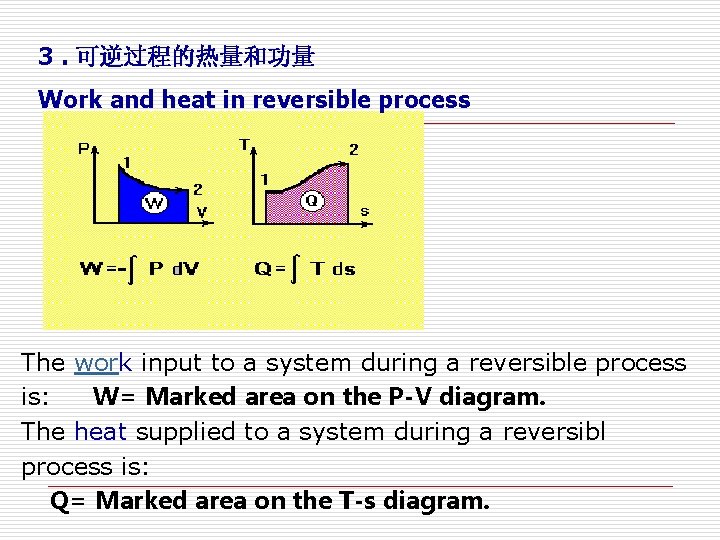
3. 可逆过程的热量和功量 Work and heat in reversible process The work input to a system during a reversible process is: W= Marked area on the P-V diagram. The heat supplied to a system during a reversibl process is: Q= Marked area on the T-s diagram.

§ 1 -5 热力循环 Cycle 要实现连续作功,必须构成循环 定义(Definition): 热力系统经过一系列变化回到初态,这一系列变 化过程称为热力循环。 A system is said to have undergone a cycle if it returns to its initial state at the end of the process
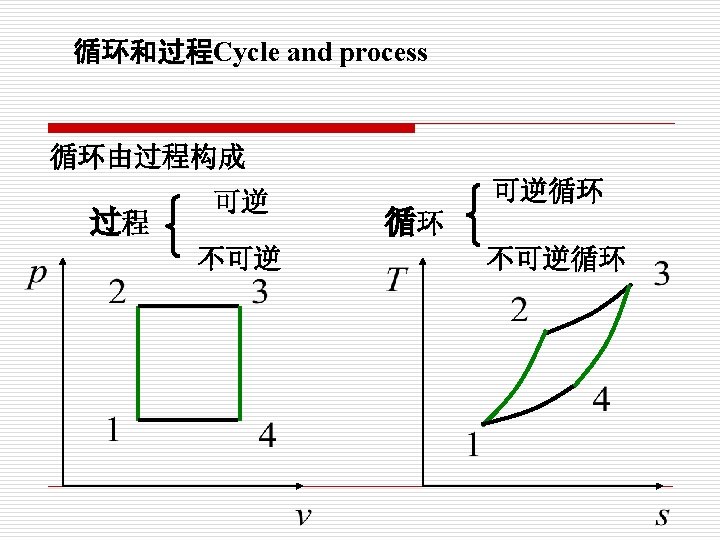
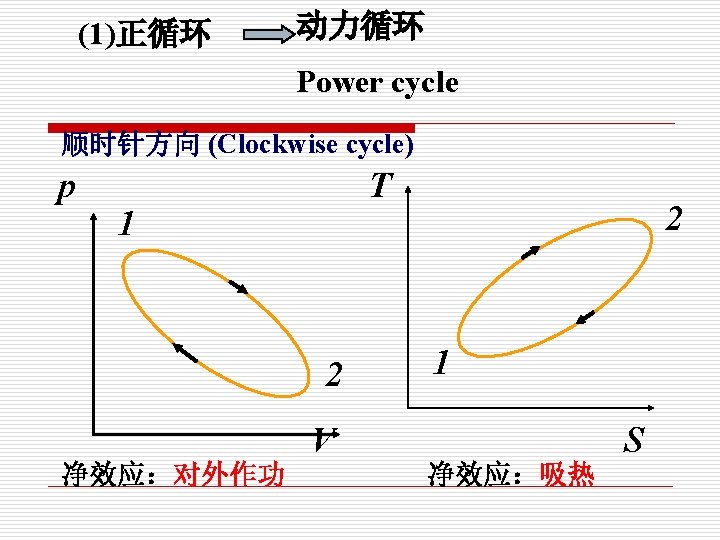
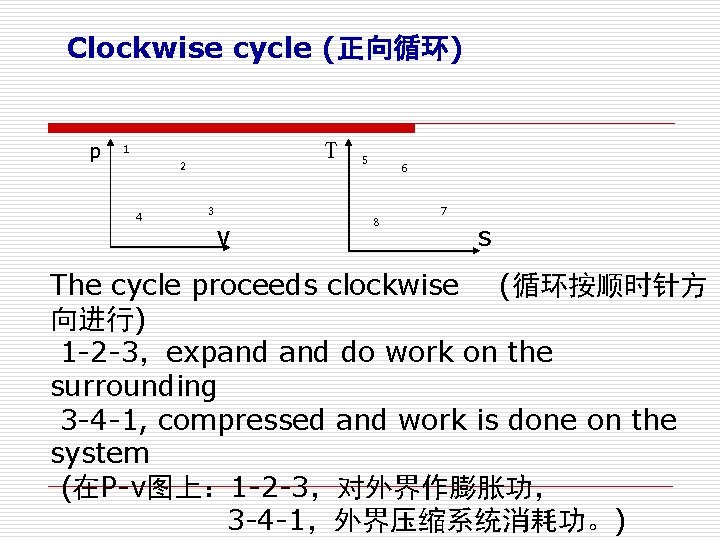
Clockwise cycle (正向循环) p T 1 2 4 3 5 6 8 7 v s The cycle proceeds clockwise (循环按顺时针方 向进行) 1 -2 -3,expand do work on the surrounding 3 -4 -1, compressed and work is done on the system (在P-v图上: 1 -2 -3,对外界作膨胀功, 3 -4 -1,外界压缩系统消耗功。)

The net work is positive and heat is converted to work. (循环净功wnet=>0), 把 热能转换为功。 在T-s图上: 5 -6 -7,absorb heat from the high temperature reservoir(从高温 热源吸 热qq ) , 7 -8 -5,reject heat to low temperature reservoir (向低温热源放热qq) The net heat flow (净热量)is qnet=qqqq, and wnet=qn
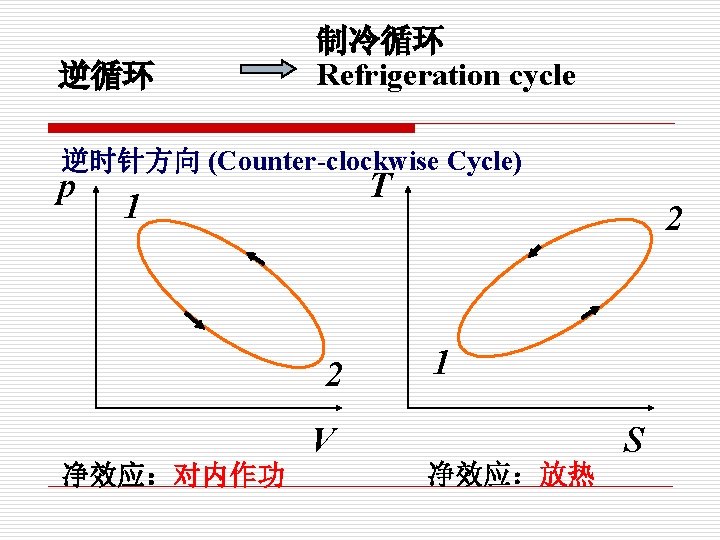
逆循环 制冷循环 Refrigeration cycle 逆时针方向 (Counter-clockwise Cycle) p T 1 2 V 净效应:对内作功 2 1 净效应:放热 S
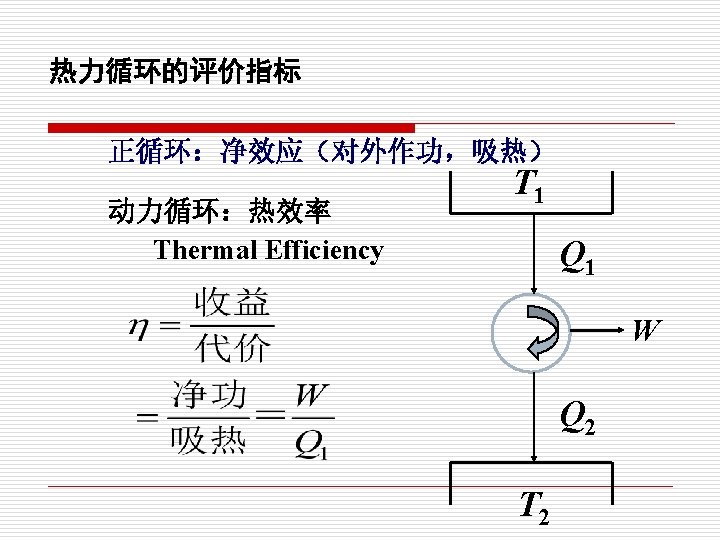
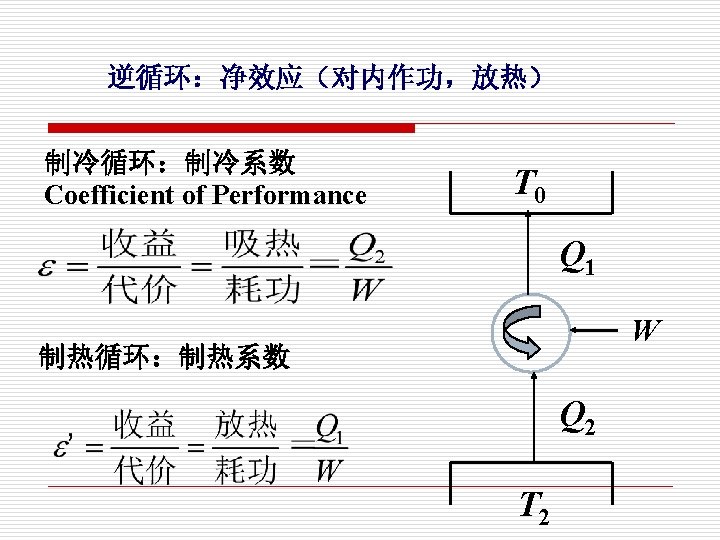
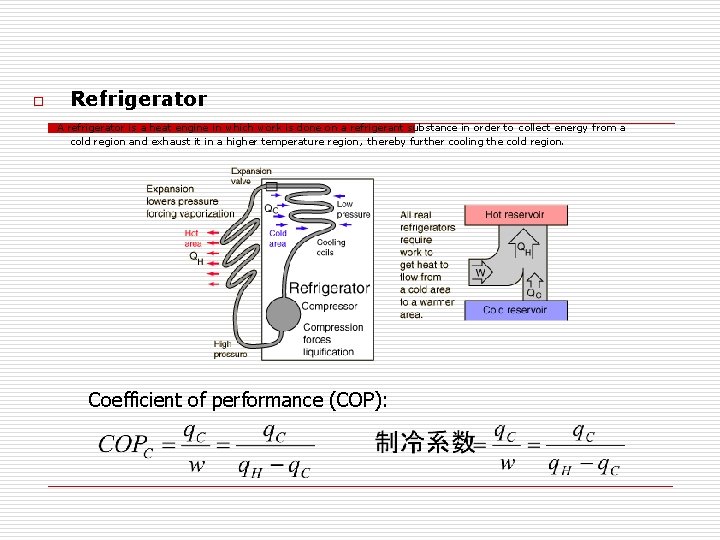
o Refrigerator A refrigerator is a heat engine in which work is done on a refrigerant substance in order to collect energy from a cold region and exhaust it in a higher temperature region, thereby further cooling the cold region. Coefficient of performance (COP):
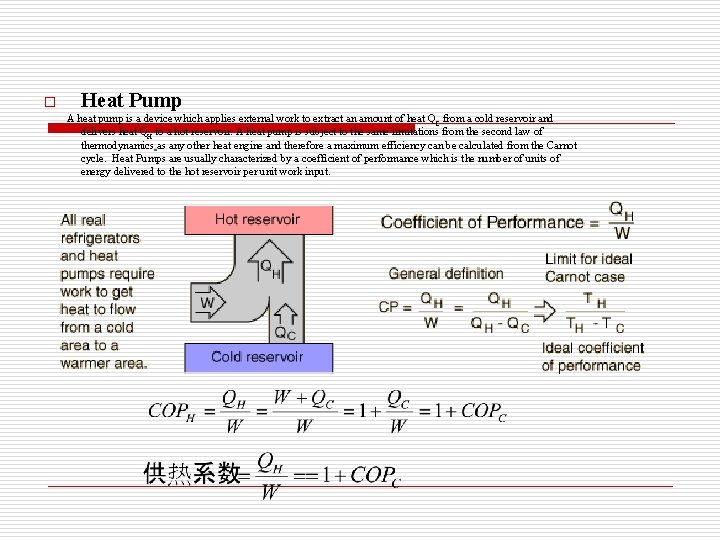
o Heat Pump A heat pump is a device which applies external work to extract an amount of heat QC from a cold reservoir and delivers heat QH to a hot reservoir. A heat pump is subject to the same limitations from the second law of thermodynamics as any other heat engine and therefore a maximum efficiency can be calculated from the Carnot cycle. Heat Pumps are usually characterized by a coefficient of performance which is the number of units of energy delivered to the hot reservoir per unit work input.
第一章小 结 Summary 热力系 System 平衡态 Equilibrium State 准静态、可逆 Quasi-static process Reversible process 过程量、状态参数 功量、热量、熵 p-V图、T-S图 循环、评价指标 Work, heat and Entropy p-V and T-S Diagram Cycle

阅读和复习 Reading and Review Book of English version (英文版教材) l Sections 1. 3~1. 6 on Page 8~14,( 第 8~14页 第 1. 3~1. 6节) l Sections 1. 10~1. 12 on Page 25~37 (第 25~37页 第 1. 10~1. 12节) l section 5. 7 on Page 265~269. (第 265~269页 5. 7节) 中文版教材 Book in (Chinese version) l 第一章 (Chapter 1)

第一章 结束 End of Chapter 1
- Slides: 113