Chapter 1 Activity 7 How Electrons Determine Chemical




- Slides: 18

Chapter 1 Activity 7: How Electrons Determine Chemical Behavior


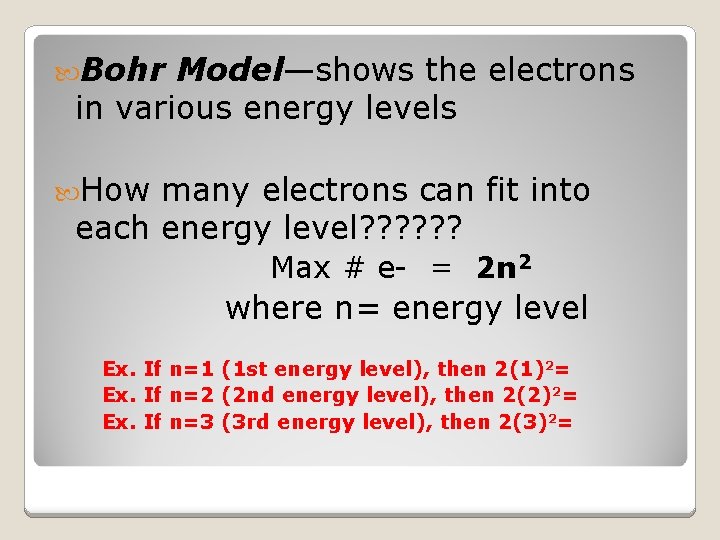
Bohr Model—shows the electrons in various energy levels How many electrons can fit into each energy level? ? ? Max # e- = 2 n 2 where n= energy level Ex. If n=1 (1 st energy level), then 2(1)2= Ex. If n=2 (2 nd energy level), then 2(2)2= Ex. If n=3 (3 rd energy level), then 2(3)2=

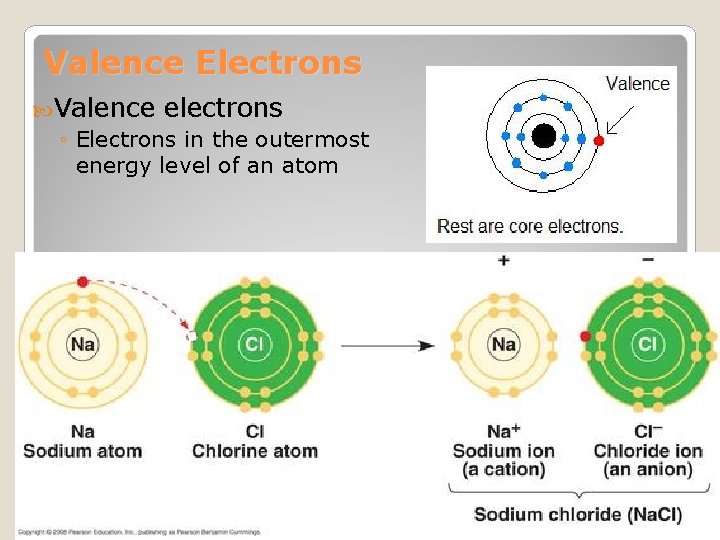
Valence Electrons Valence electrons ◦ Electrons in the outermost energy level of an atom

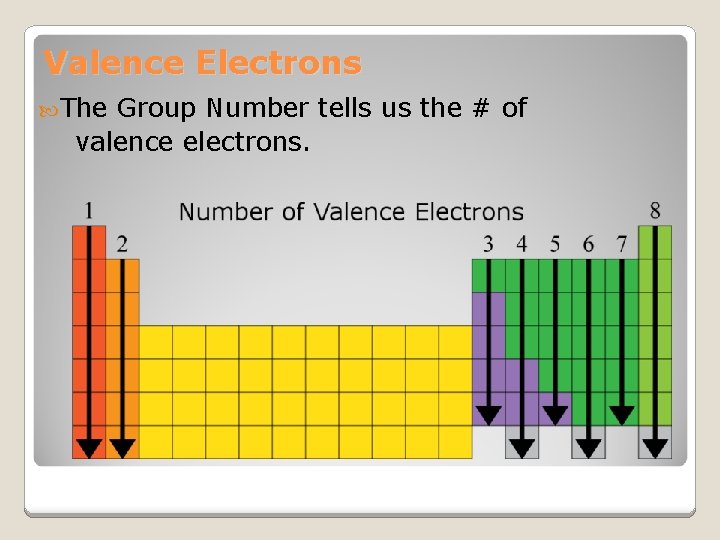
Valence Electrons The Group Number tells us the # of valence electrons.

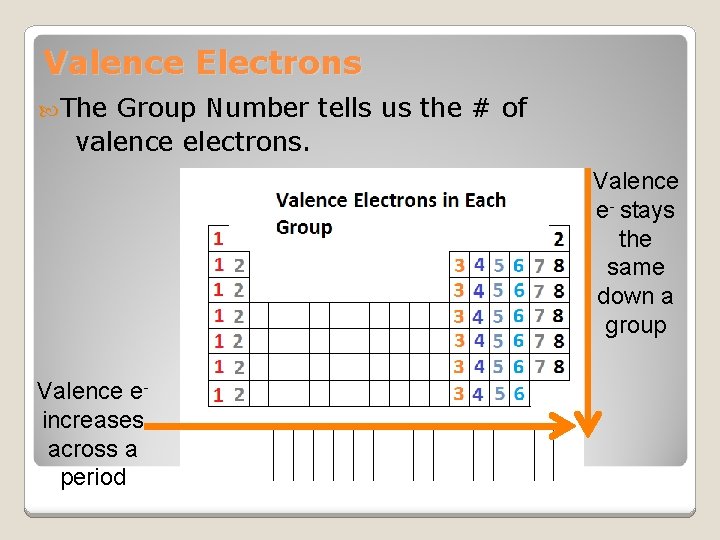
Valence Electrons The Group Number tells us the # of valence electrons. Valence e- stays the same down a group Valence e- increases across a period

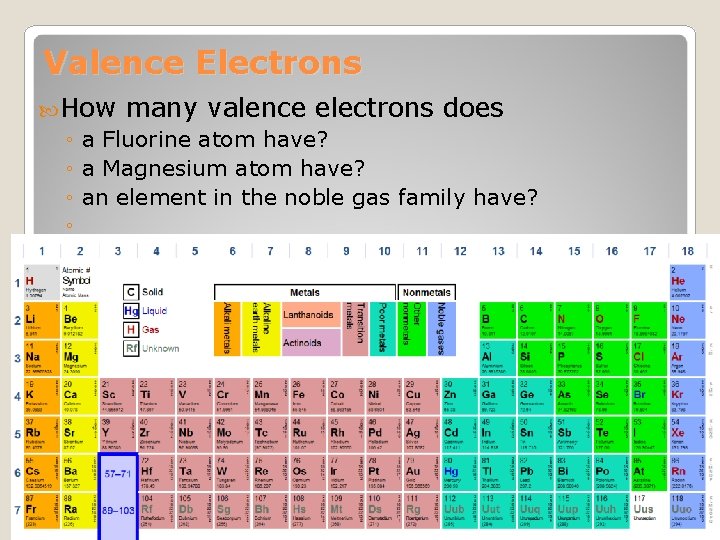
Valence Electrons How many valence electrons does ◦ a Fluorine atom have? ◦ a Magnesium atom have? ◦ an element in the noble gas family have? ◦

Things You Need to Now Know On the periodic table: ◦ The COLUMN or GROUP or FAMILY equals the # of valence electrons (for columns 1, 2, 13 -18) ◦ The ROW or PERIOD equals the energy level

Bohr Models Rutherford focused on describing the nucleus Bohr focused on describing the location of the electrons Bohr model (planetary model): -shows electrons moving in orbits (energy levels) around the positive nucleus of the atom.

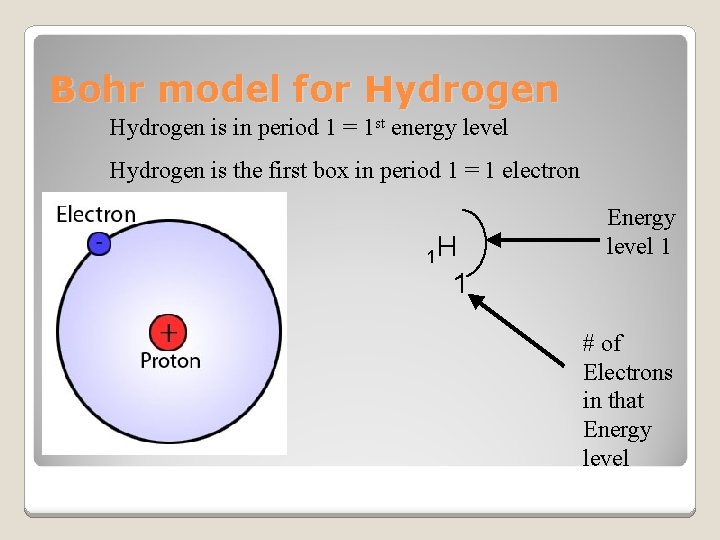
Bohr model for Hydrogen is in period 1 = 1 st energy level Hydrogen is the first box in period 1 = 1 electron 1 H Energy level 1 1 # of Electrons in that Energy level

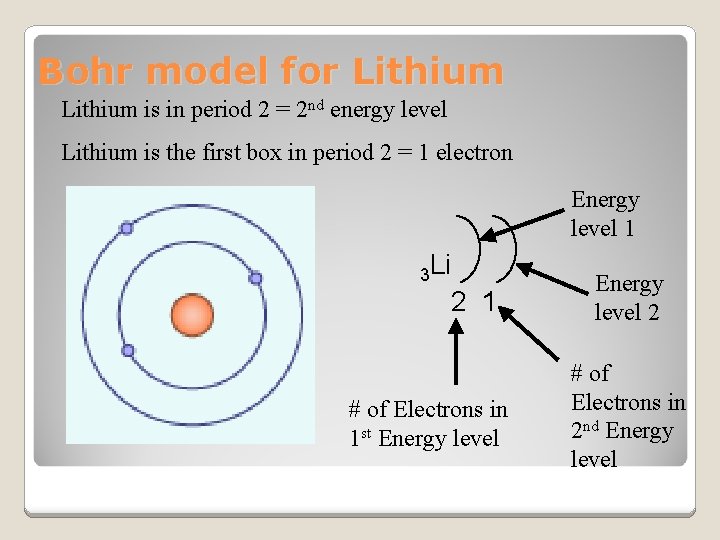
Bohr model for Lithium is in period 2 = 2 nd energy level Lithium is the first box in period 2 = 1 electron Energy level 1 3 Li 2 1 Energy level 2 # of Electrons in 1 st Energy level # of Electrons in 2 nd Energy level

Using What You Now Know Duet Rule: In forming compounds, atoms tend to gain or lose electrons in order to have two electrons in their first energy level. Octet Rule: In forming compounds, atoms tend to gain or lose electrons in order to have eight electrons in their outer energy level.

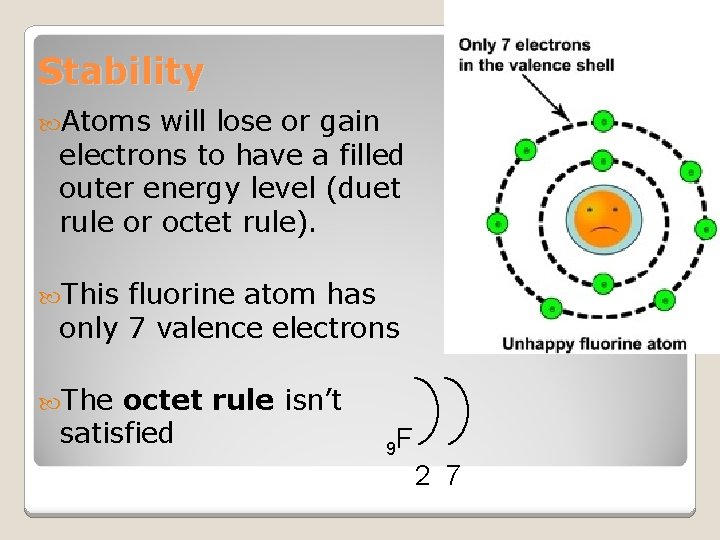
Stability Atoms will lose or gain electrons to have a filled outer energy level (duet rule or octet rule). This fluorine atom has only 7 valence electrons The octet rule isn’t satisfied 9 F 2 7

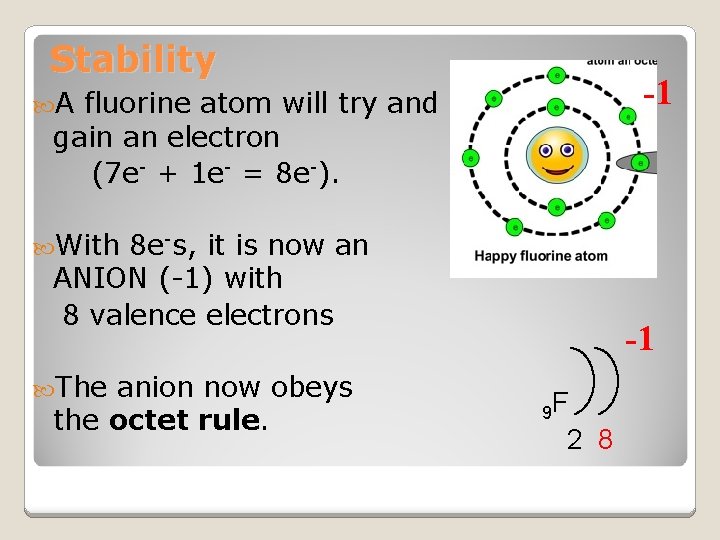
Stability -1 A fluorine atom will try and gain an electron (7 e- + 1 e- = 8 e-). With 8 e-s, it is now an ANION (-1) with 8 valence electrons The anion now obeys the octet rule. -1 9 F 2 8

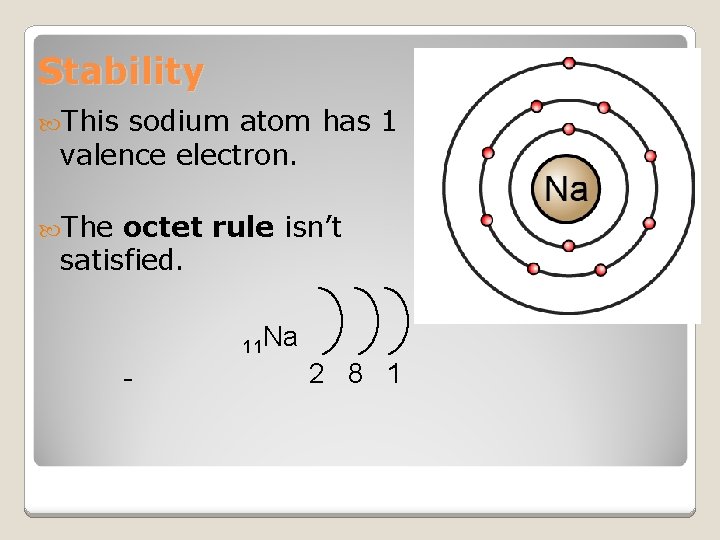
Stability This sodium atom has 1 valence electron. The octet rule isn’t satisfied. 11 Na 2 8 1

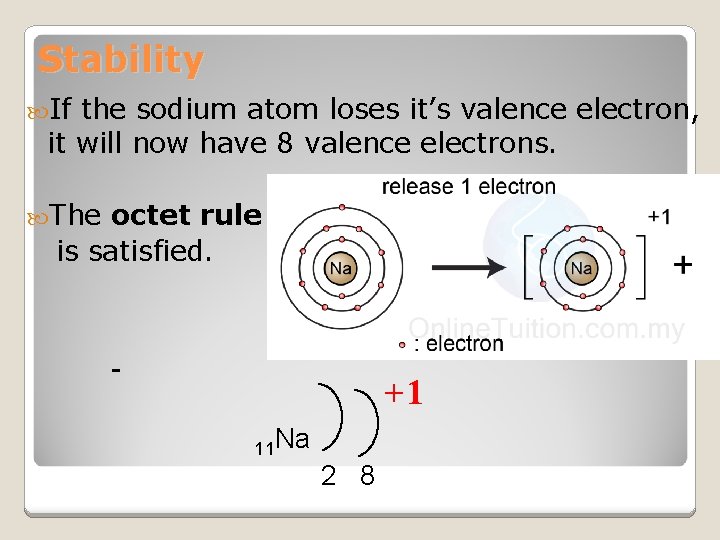
Stability If the sodium atom loses it’s valence electron, it will now have 8 valence electrons. The octet rule is satisfied. +1 11 Na 2 8

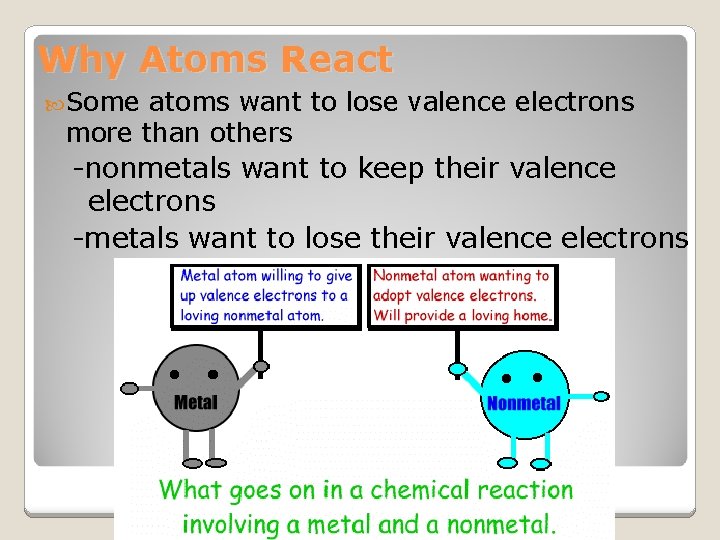
Why Atoms React Some atoms want to lose valence electrons more than others -nonmetals want to keep their valence electrons -metals want to lose their valence electrons

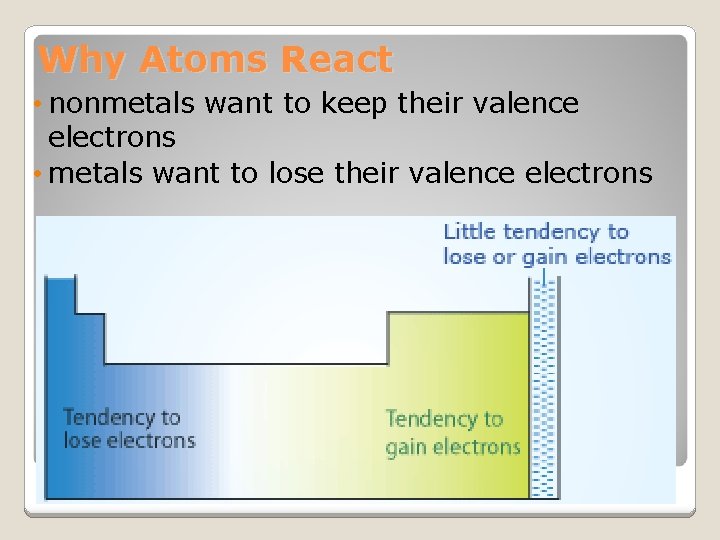
Why Atoms React • nonmetals want to keep their valence electrons • metals want to lose their valence electrons