Chapter 1 2 Describing Matter Physical Science 1

Chapter 1 -2 Describing Matter Physical Science

1 -2 Describing Matter n Objectives n 1. Give examples of matter's different properties. n 2. Describe how density is used to identify different substances. n 3. Compare physical and chemical properties. n 4. Explain what happens to matter during physical and chemical changes. n New Terms n physical property n physical change n Density n chemical change n chemical property

Physical Properties n Something that can be observed or measured without changing the identity of the matter.

Examples of Physical Properties n 1. color n 2. odor n 3. volume n 4. mass n 5. thermal conductivity - ability to transform thermal energy from one area to another. n 6. State - solid, liquid, gas n 7. Malleability - ability to be pounded into thin sheets n 8. Ductility - ability to be drawn or pulled into a wire n 9. Solubility - ability to dissolve in another substance n 10. Density - mass per unit volume

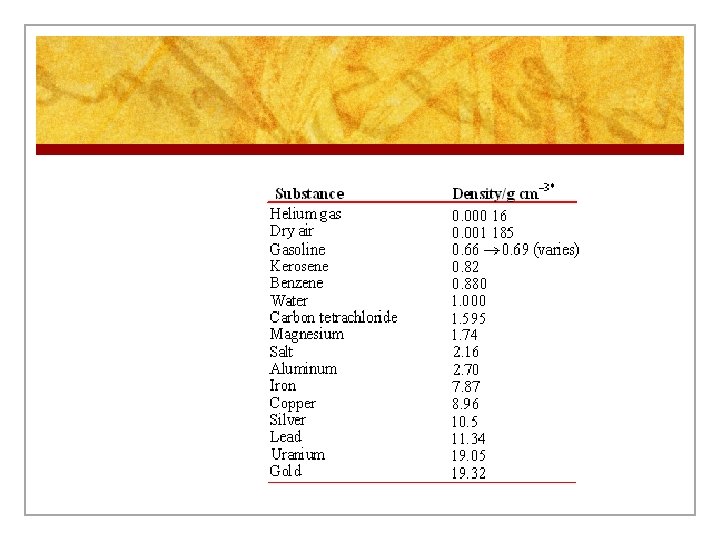
n Density is the amount of matter in a given volume. n D = m/v and the units are g/cm 3, g/m. L, kg/m 3, or kg/L n Density helps us be able to identify substances because the density of a specific substance is always the same at the same pressure and temperature. Also the density of one substance is different from any other substance.

Liquid Layers n Liquids will separate by density. The densest will go the bottom and the least dense will be the one at the top. n If we shook this up would it blend together? n Density does not matter on the amount of the substance.

Chemical Properties n Describe a substance based on its ability to change into a new substance with different properties. Example: burning a piece of wood, flammability. n Other chemical properties would be: reactivity with oxygen, reactivity with acid, reactivity with water.

Physical Changes n A change that affects one or more physical properties. For example - breaking chalk, melting a substance, dissolving a substance, freezing, sanding a piece of wood, bending a paper clip, cutting your hair. n Can physical changes be undone? Most of the time they can be undone.

Chemical Changes n Chemical Changes - when one or more substances are changed into entirely new substances. The new substances have completely different properties from the original. n Examples: baking a cake, soured milk, hot gas formed from a space shuttle blast, the Statue of Liberty being green, using Alka-Seltzer tablets.

n Chemical changes often cause color changes, fizzing or foaming, production of heat, sound, light, or odor. n Can a chemical change be undone - no because you are making a new substance.
- Slides: 11