Chapter 06 Interactions Between Cells and the Extracellular
Chapter 06 Interactions Between Cells and the Extracellular Environment Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

1. Extracellular Environment
Introduction 1. The extracellular environment includes everything located outside the cells. 2. Cells receive nourishment from and release wastes into the extracellular environment. 3. Cells communicate with each other by secreting chemical regulators into the extracellular environment.
Body Fluids 1. 67% of our water is within cells in the intracellular compartment. 2. 33% is in the extracellular compartment. Of this: 1. 20% is in blood plasma. 2. 80% makes up what is called tissue fluid, or interstitial fluid; connects the intracellular compartment with the blood plasma.
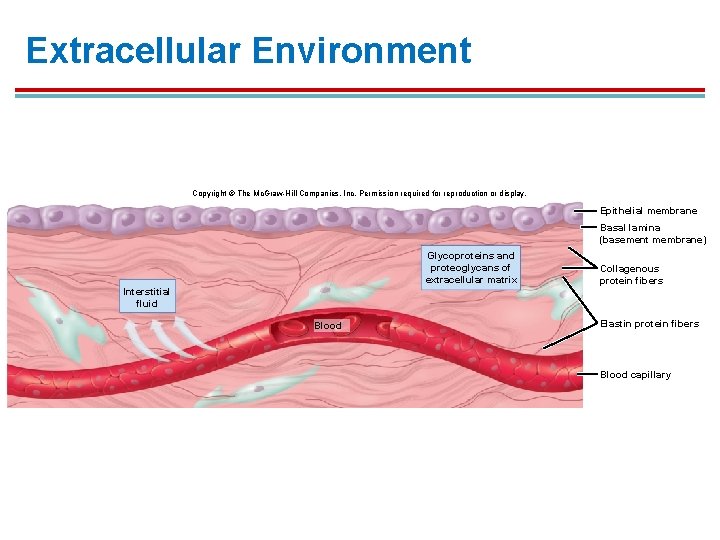
Extracellular Environment Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Epithelial membrane Basal lamina (basement membrane) Glycoproteins and proteoglycans of extracellular matrix Interstitial fluid Blood Collagenous protein fibers Elastin protein fibers Blood capillary
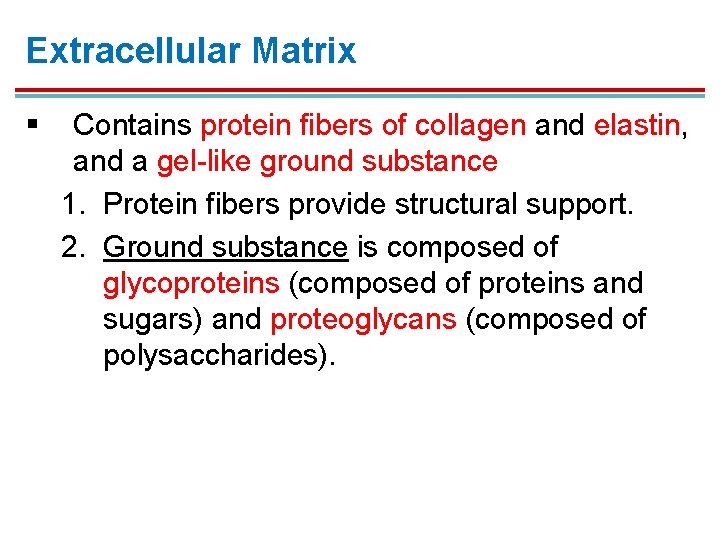
Extracellular Matrix § Contains protein fibers of collagen and elastin, and a gel-like ground substance 1. Protein fibers provide structural support. 2. Ground substance is composed of glycoproteins (composed of proteins and sugars) and proteoglycans (composed of polysaccharides).
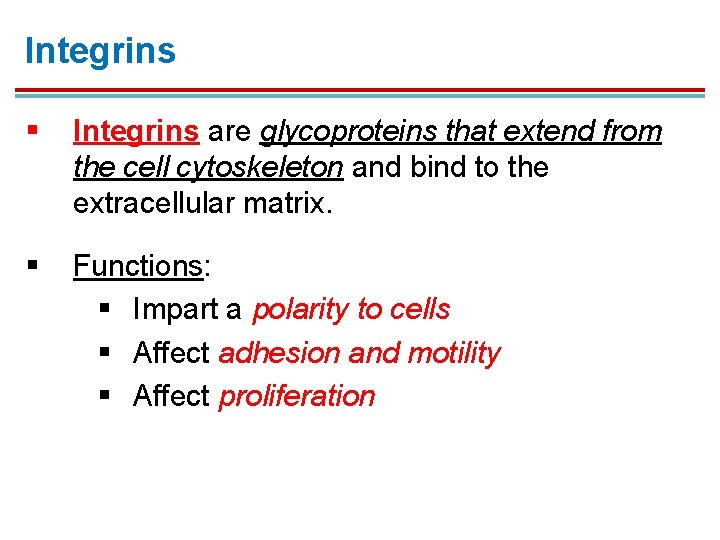
Integrins § Integrins are glycoproteins that extend from the cell cytoskeleton and bind to the extracellular matrix. § Functions: § Impart a polarity to cells § Affect adhesion and motility § Affect proliferation
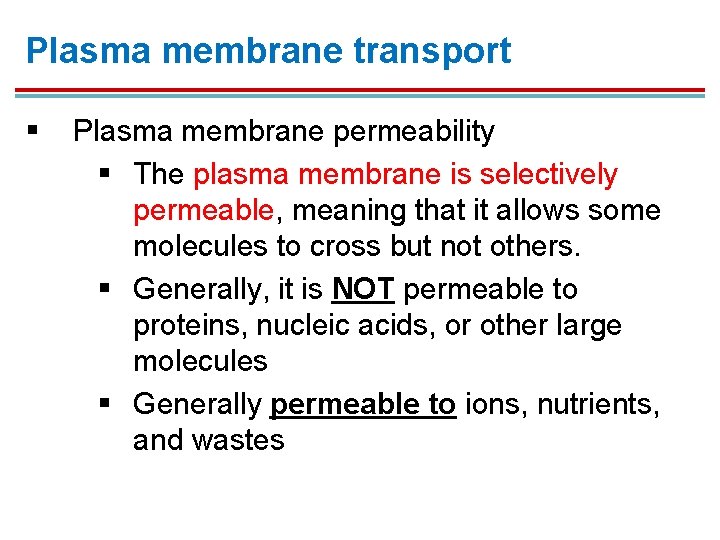
Plasma membrane transport § Plasma membrane permeability § The plasma membrane is selectively permeable, meaning that it allows some molecules to cross but not others. § Generally, it is NOT permeable to proteins, nucleic acids, or other large molecules § Generally permeable to ions, nutrients, and wastes
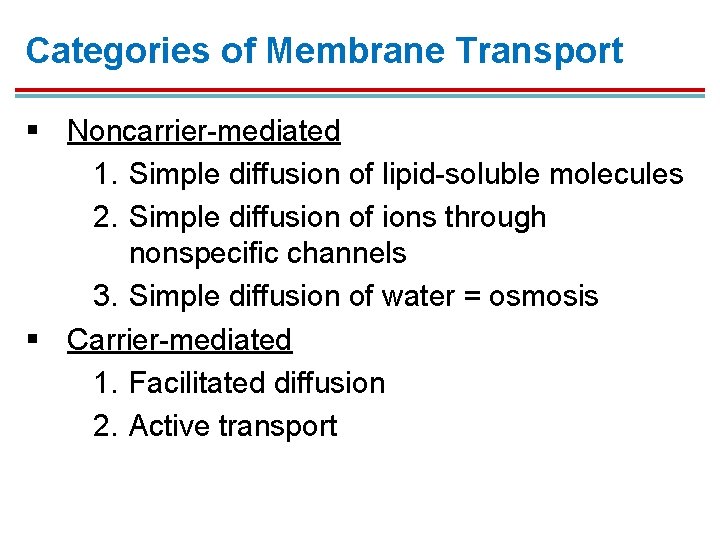
Categories of Membrane Transport § Noncarrier-mediated 1. Simple diffusion of lipid-soluble molecules 2. Simple diffusion of ions through nonspecific channels 3. Simple diffusion of water = osmosis § Carrier-mediated 1. Facilitated diffusion 2. Active transport
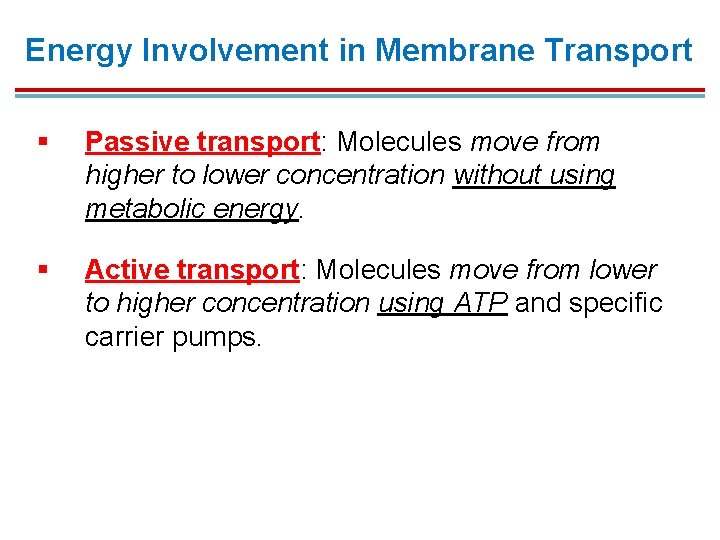
Energy Involvement in Membrane Transport § Passive transport: Molecules move from higher to lower concentration without using metabolic energy. § Active transport: Molecules move from lower to higher concentration using ATP and specific carrier pumps.
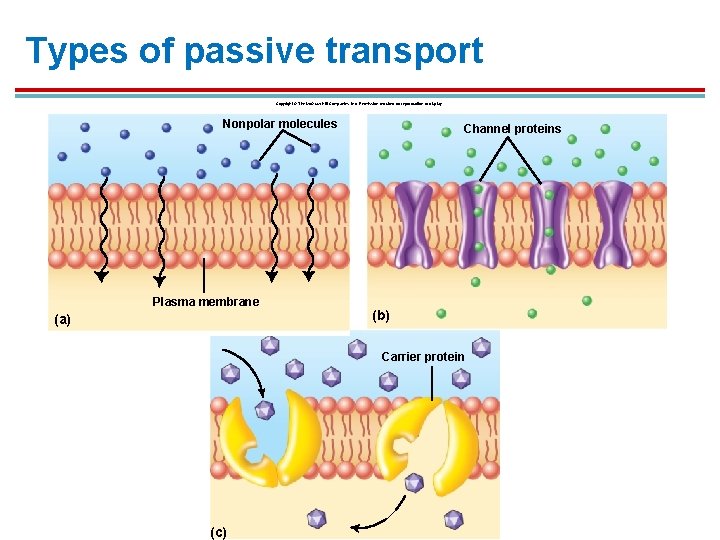
Types of passive transport Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Nonpolar molecules Plasma membrane (a) Channel proteins (b) Carrier protein (c)
1. Diffusion and Osmosis
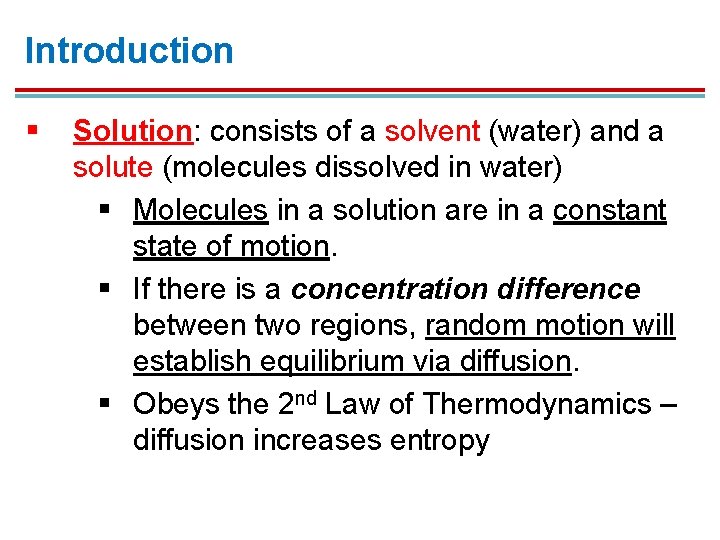
Introduction § Solution: consists of a solvent (water) and a solute (molecules dissolved in water) § Molecules in a solution are in a constant state of motion. § If there is a concentration difference between two regions, random motion will establish equilibrium via diffusion. § Obeys the 2 nd Law of Thermodynamics – diffusion increases entropy
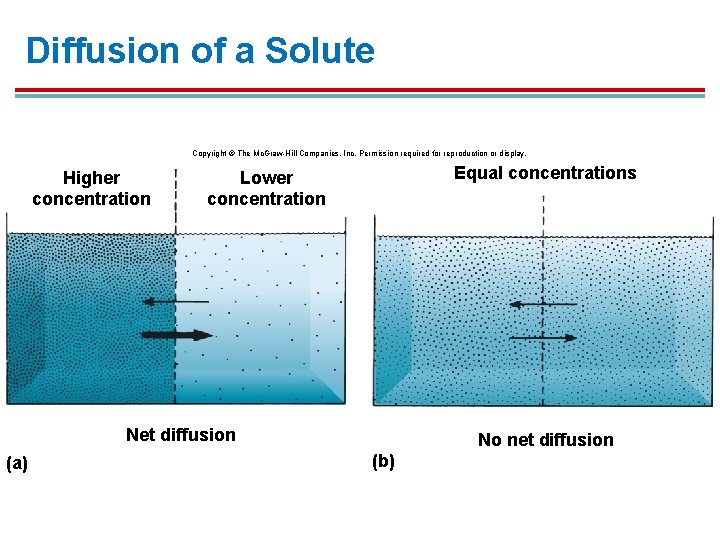
Diffusion of a Solute Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Higher concentration Equal concentrations Lower concentration Net diffusion (a) No net diffusion (b)
Introduction § § § Diffusion will occur without a physical separation or across a permeable membrane Net diffusion - due to random movement, the net direction of diffusion is from high to low solute concentration. Mean diffusion time – the average time it takes for a solute to diffuse 1. Increases with the square of the distance the solute must travel (less distance, faster) 2. Distances beyond 100 µm, diffusion time is too long to be effective
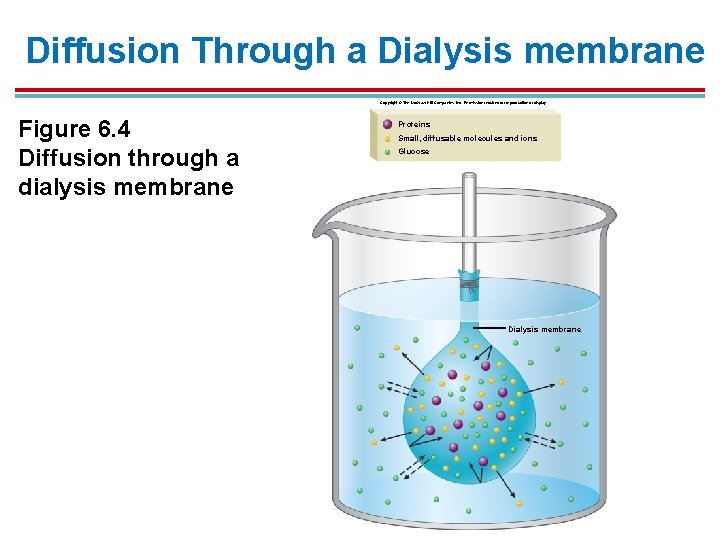
Diffusion Through a Dialysis membrane Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Figure 6. 4 Diffusion through a dialysis membrane Proteins Small, diffusable molecules and ions Glucose Dialysis membrane
Diffusion Through the Plasma Membrane § § § Small, nonpolar (or uncharged) lipid-soluble molecules pass easily through the lipid portion of the membrane. Includes: 1. Oxygen, carbon dioxide, and steroid hormones Gas exchange: net diffusion of O 2 and CO 2 whether in and out of cells or from air to blood is due to concentration gradients (high low) Water can pass through using special channels called aquaporins in a process called osmosis
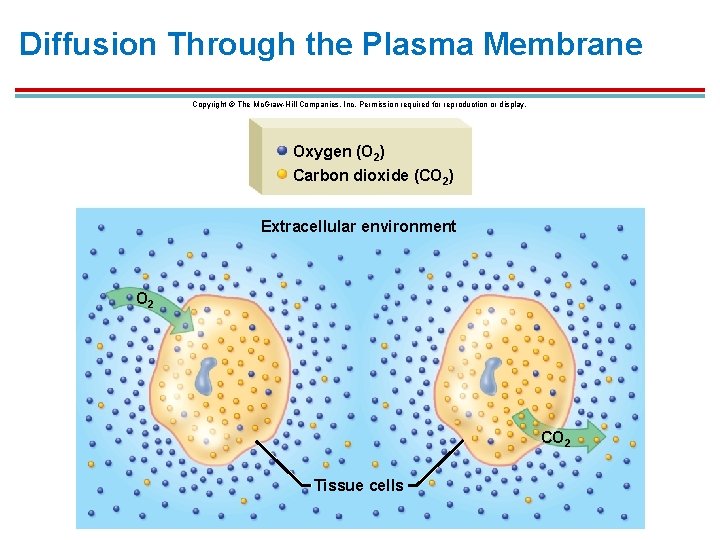
Diffusion Through the Plasma Membrane Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Oxygen (O 2) Carbon dioxide (CO 2) Extracellular environment O 2 CO 2 Tissue cells
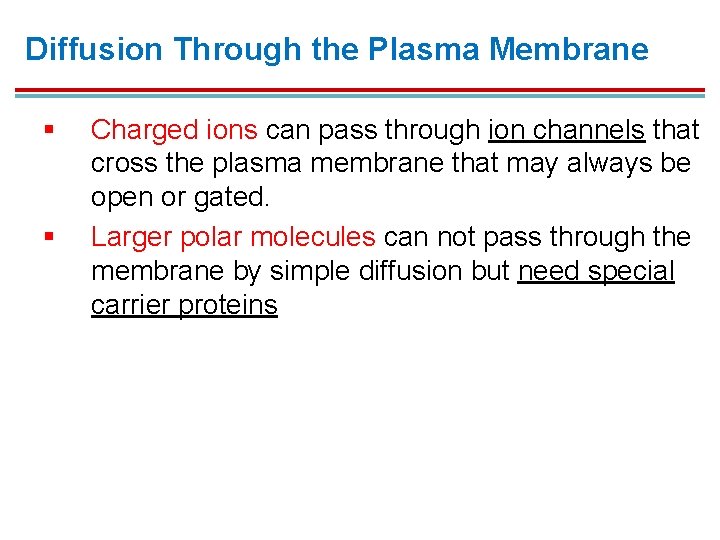
Diffusion Through the Plasma Membrane § § Charged ions can pass through ion channels that cross the plasma membrane that may always be open or gated. Larger polar molecules can not pass through the membrane by simple diffusion but need special carrier proteins
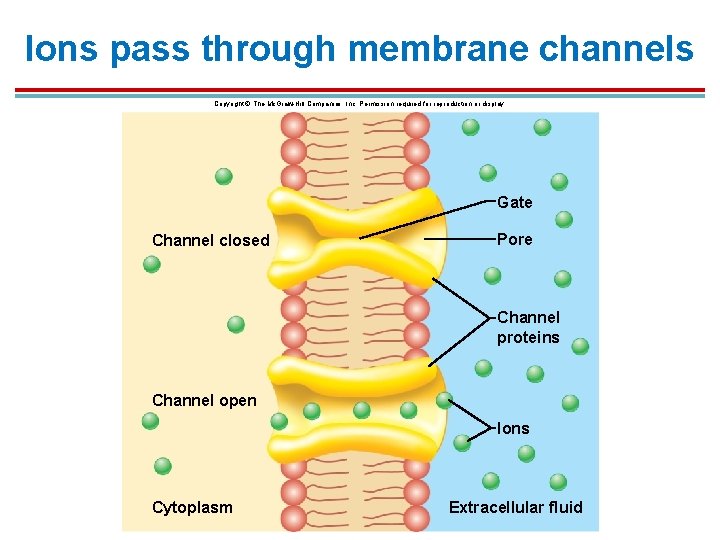
Ions pass through membrane channels Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Gate Channel closed Pore Channel proteins Channel open Ions Cytoplasm Extracellular fluid
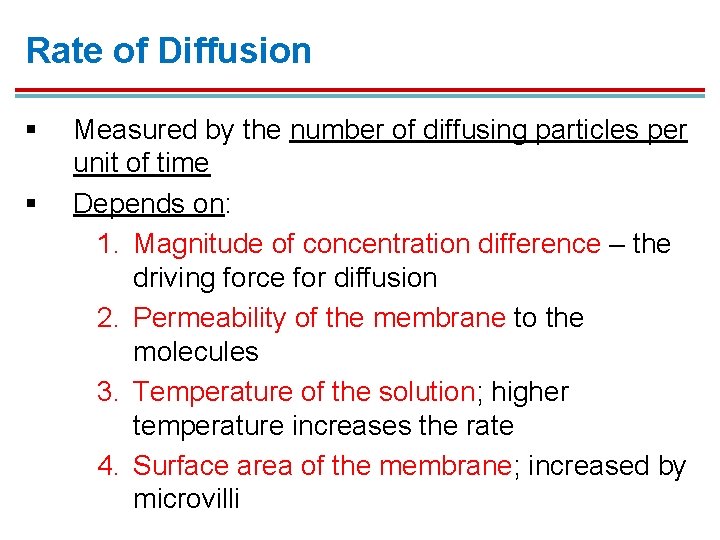
Rate of Diffusion § § Measured by the number of diffusing particles per unit of time Depends on: 1. Magnitude of concentration difference – the driving force for diffusion 2. Permeability of the membrane to the molecules 3. Temperature of the solution; higher temperature increases the rate 4. Surface area of the membrane; increased by microvilli

Osmosis § § Water molecules do not carry a charge, so they can pass through the plasma membrane slowly. This is the diffusion of solvent instead of solute, it is unique. 1. Given a special name - osmosis 2. Aided by channels in the membrane called aquaporins 3. Many aquaporins are found in the kidneys, eyes, lungs, salivary glands, and the brain
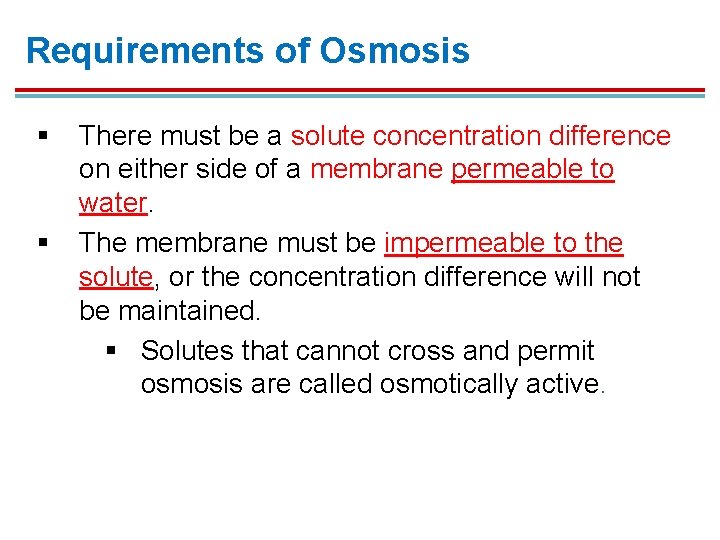
Requirements of Osmosis § § There must be a solute concentration difference on either side of a membrane permeable to water. The membrane must be impermeable to the solute, or the concentration difference will not be maintained. § Solutes that cannot cross and permit osmosis are called osmotically active.
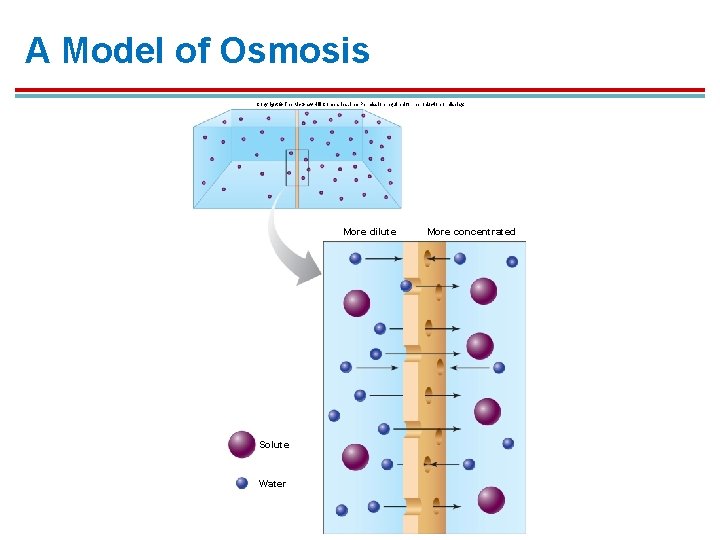
A Model of Osmosis Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. More dilute Solute Water More concentrated
Water movement in Osmosis § § The net movement of water is from the side with more water (more dilute) to the side with less water (less dilute). - or Water moves from the area of low solute concentration to the area of high solute concentration. (water moves toward the side with the higher solute concentration)
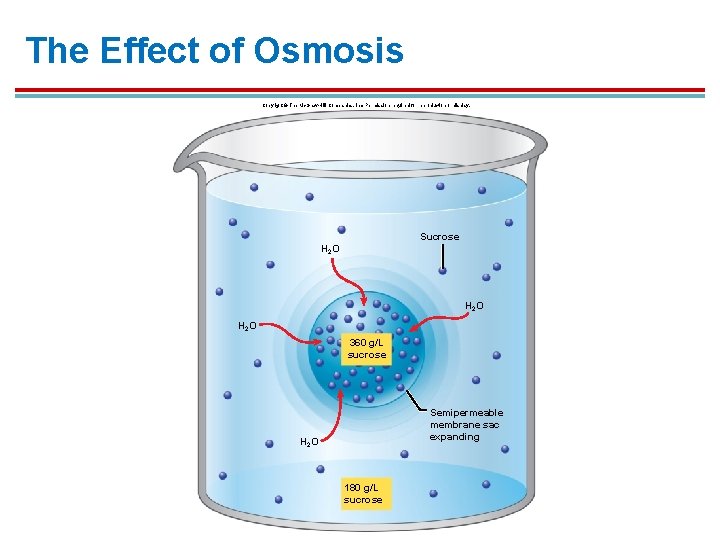
The Effect of Osmosis Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Sucrose H 2 O 360 g/L sucrose Semipermeable membrane sac expanding H 2 O 180 g/L sucrose

Osmotic Pressure § § § Osmotic pressure is the force surrounding a cell required to stop osmosis. Can be used to describe the osmotic pull of a solution. A higher solute concentration would require a higher osmotic pressure. § Pure water has an osmotic pressure of zero Osmotic pressure depends on the ratio of solute to solvent.
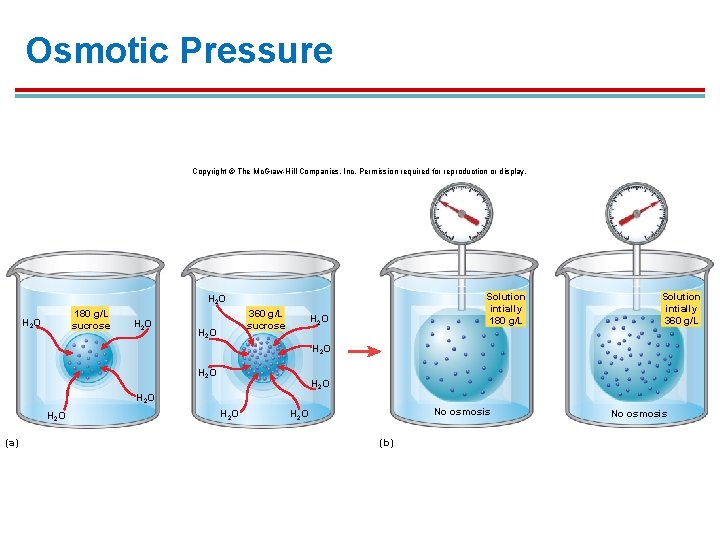
Osmotic Pressure Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Solution intially 180 g/L H 2 O 180 g/L sucrose H 2 O 360 g/L sucrose H 2 O Solution intially 360 g/L H 2 O H 2 O (a) H 2 O No osmosis H 2 O (b) No osmosis
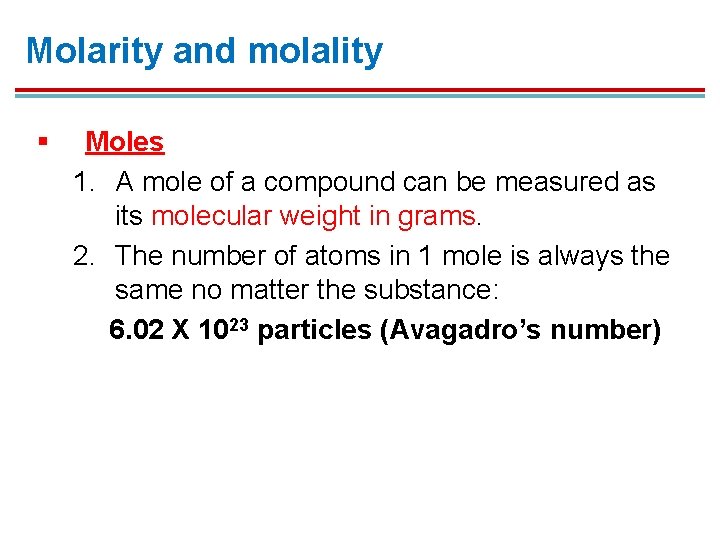
Molarity and molality § Moles 1. A mole of a compound can be measured as its molecular weight in grams. 2. The number of atoms in 1 mole is always the same no matter the substance: 6. 02 X 1023 particles (Avagadro’s number)

Molarity – moles solute/Liter solution § To make a Molar solution, take the weight in grams of that solute and dissolve in enough water to make a 1 L solution = 1. 0 M solution of that solute § § To make a 1. 0 molar (M) solution of glucose, dissolve 180 g glucose in water to make 1 L solution. To make a 1. 0 M solution of Na. Cl, dissolve 58. 5 g Na. Cl in water to make 1 L solution.
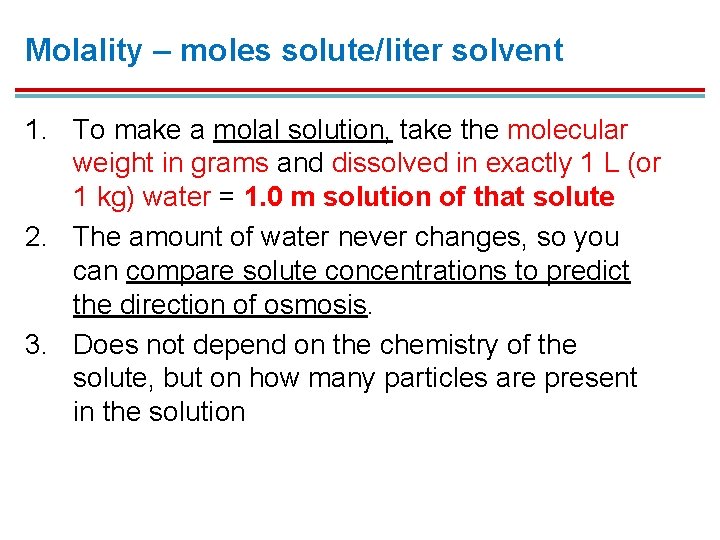
Molality – moles solute/liter solvent 1. To make a molal solution, take the molecular weight in grams and dissolved in exactly 1 L (or 1 kg) water = 1. 0 m solution of that solute 2. The amount of water never changes, so you can compare solute concentrations to predict the direction of osmosis. 3. Does not depend on the chemistry of the solute, but on how many particles are present in the solution
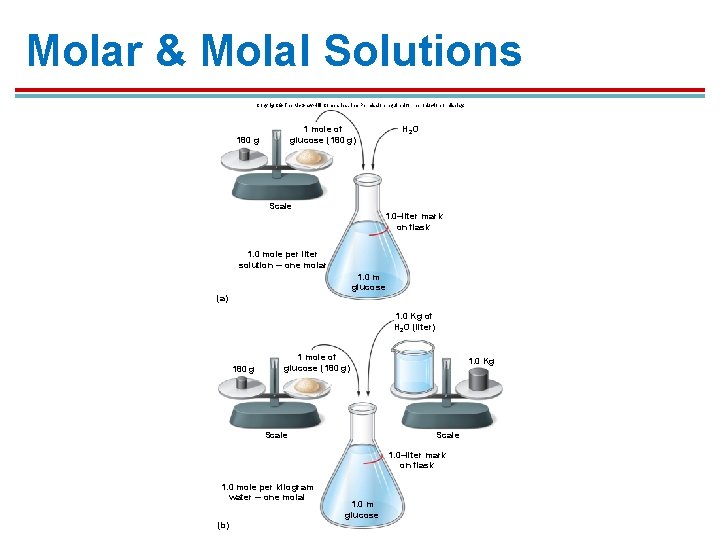
Molar & Molal Solutions Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H 2 O 1 mole of glucose (180 g) 180 g Scale 1. 0–liter mark on flask 1. 0 mole per liter solution ─ one molar 1. 0 m glucose (a) 1. 0 Kg of H 2 O (liter) 180 g 1 mole of glucose (180 g) Scale 1. 0 Kg Scale 1. 0–liter mark on flask 1. 0 mole per kilogram water ─ one molal (b) 1. 0 m glucose
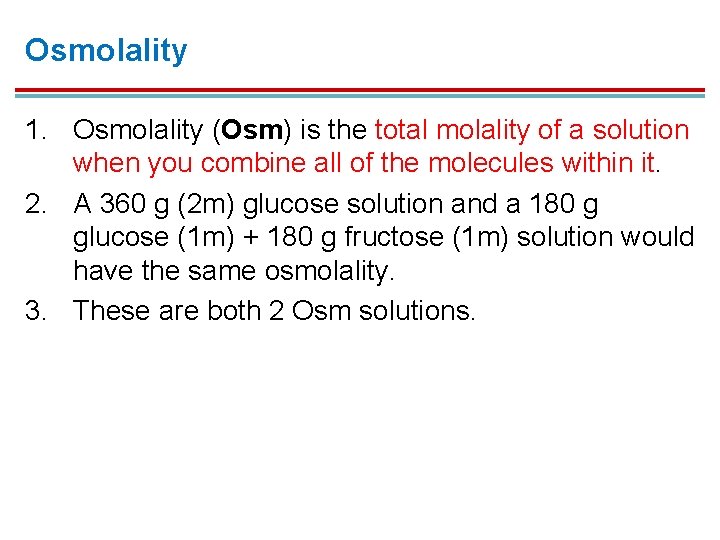
Osmolality 1. Osmolality (Osm) is the total molality of a solution when you combine all of the molecules within it. 2. A 360 g (2 m) glucose solution and a 180 g glucose (1 m) + 180 g fructose (1 m) solution would have the same osmolality. 3. These are both 2 Osm solutions.
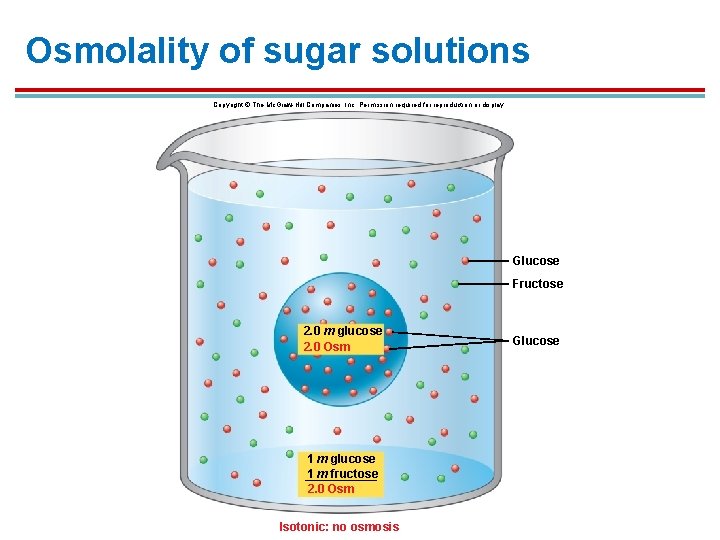
Osmolality of sugar solutions Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Glucose Fructose 2. 0 m glucose 2. 0 Osm 1 m glucose 1 m fructose 2. 0 Osm Isotonic: no osmosis Glucose
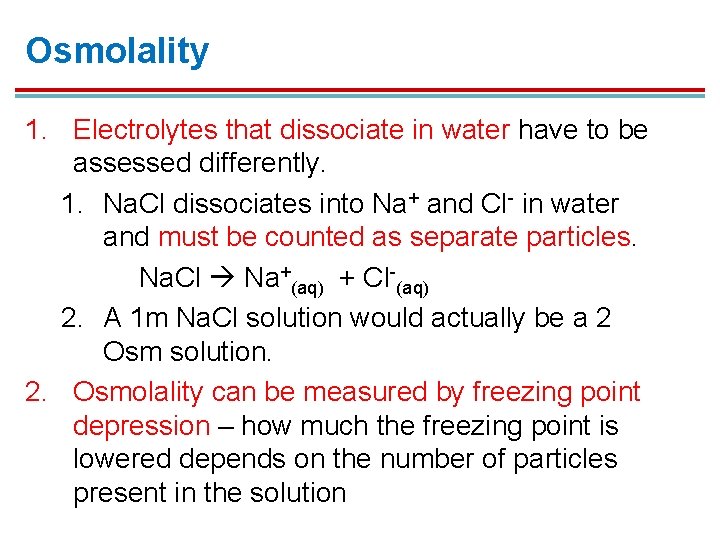
Osmolality 1. Electrolytes that dissociate in water have to be assessed differently. 1. Na. Cl dissociates into Na+ and Cl- in water and must be counted as separate particles. Na. Cl Na+(aq) + Cl-(aq) 2. A 1 m Na. Cl solution would actually be a 2 Osm solution. 2. Osmolality can be measured by freezing point depression – how much the freezing point is lowered depends on the number of particles present in the solution
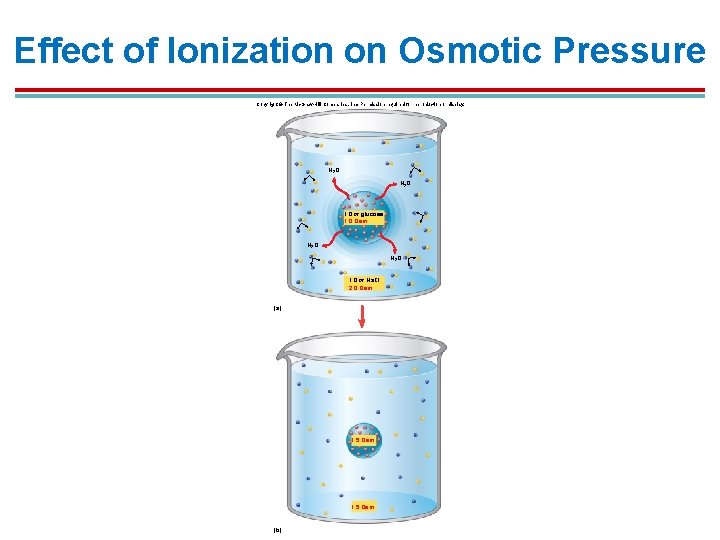
Effect of Ionization on Osmotic Pressure Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H 2 O 1. 0 m glucose 1. 0 Osm H 2 O 1. 0 m Na. Cl 2. 0 Osm (a) 1. 5 Osm (b)
Tonicity § Plasma has the same osmolality as a 0. 3 m glucose or a 0. 15 m Na. Cl solution. § § These solutions are considered isosmotic to plasma. Made as 0. 9 g Na. Cl/100 m. L water – normal saline 5% dextrose – 5 g glucose/100 m. L water Tonicity is the effect of a solute concentration on the osmotic movement of water. § If a membrane separates a 0. 3 m glucose solution and a 0. 15 m Na. Cl solution, there will be no net movement of water = isotonic.

Tonicity § Tonicity takes into account the permeability of the membrane to the solutes. If the solutes can cross the membrane, the tonicity will change, since it will cause an osmotic movement of water towards it. § RBCs placed in a solution of urea. Urea can easily diffuse through the membrane of RBCs, which increases the tone inside the cell. This will increase the osmotic pressure inside the cell to pull in water. Cell will swell and eventually bursts.

Tonicity § Solutions with a lower solute concentration than the cell are hypo-osmotic and hypotonic. § Water will be pulled into the cell; cell will swell and could lyse § Solutions with a higher solute concentration than the cell are hyper-osmotic and hypertonic. § Water will be pulled out of the cell; cell will shrivel up and could crenate
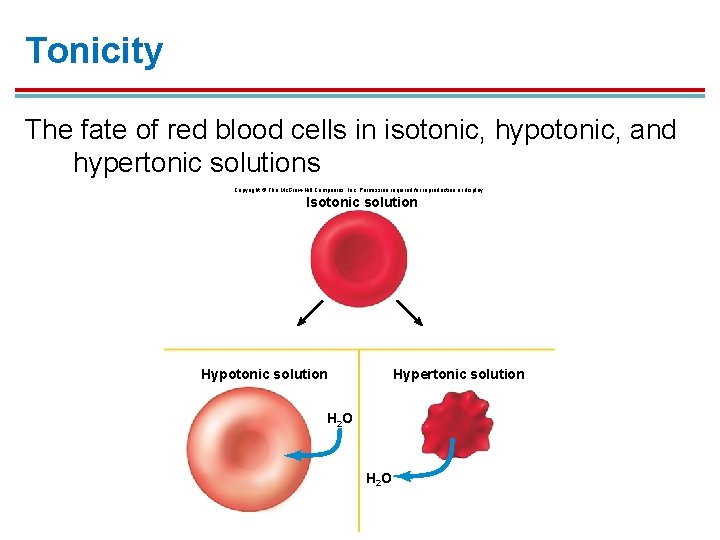
Tonicity The fate of red blood cells in isotonic, hypotonic, and hypertonic solutions Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Isotonic solution Hypertonic solution H 2 O
Regulation of Blood Osmolality § § § Constant osmolality must be maintained, or neurons will be damaged. Osmoreceptors in the hypothalamus detect increases in osmolality (due to dehydration). This triggers: 1. Thirst 2. Decreased excretion of water in urine With a lower plasma osmolality, osmoreceptors are not stimulated, so more water is excreted in urine
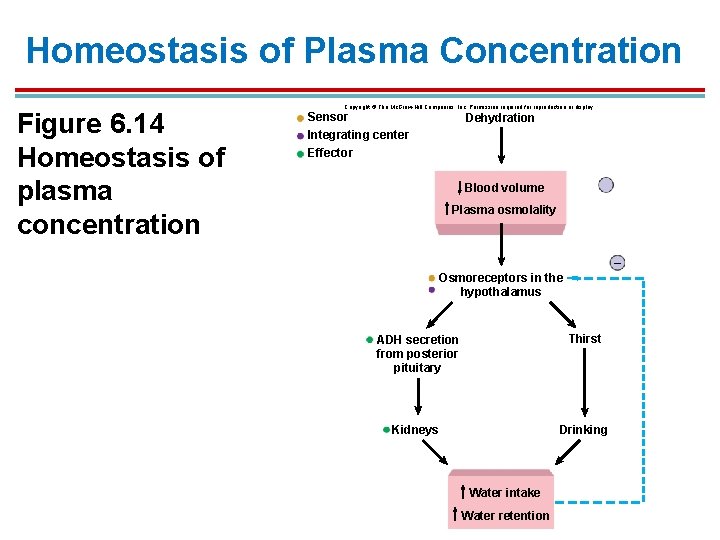
Homeostasis of Plasma Concentration Figure 6. 14 Homeostasis of plasma concentration Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Sensor Dehydration Integrating center Effector Blood volume Plasma osmolality – Osmoreceptors in the hypothalamus ADH secretion from posterior pituitary Thirst Kidneys Drinking Water intake Water retention

1. Carrier-Mediated Transport

Introduction 1. Molecules that are large or polar cannot diffuse across the membrane. 2. Includes amino acids, glucose, and other organic molecules 3. Carrier proteins within the plasma membrane move these molecules across. 4. Characteristics of the carriers 1. They are specific to a given molecule. 2. The may be competition for similar carriers or molecules 3. Saturation – number of carriers is limited

Introduction 1. Some proteins can transport more than one molecule, but then there is a competition effect. 2. Transport rates increase with increased molecule concentration until saturation is met; this is the transport maximum (Tm) where all carriers are in use
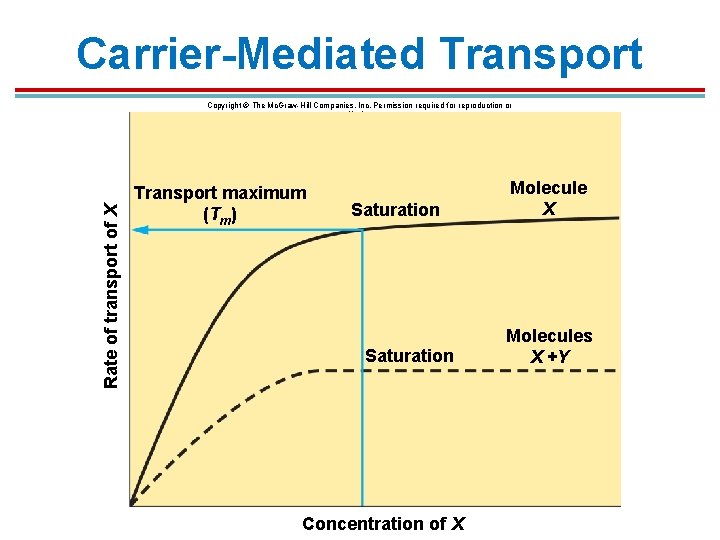
Carrier-Mediated Transport Rate of transport of X Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Transport maximum (Tm) Saturation Concentration of X Molecules X +Y
Facilitated Diffusion § § Powered by the random movement of molecules NO ATP used Net movement from high to low concentration Requires specific carrier-mediated proteins Transport proteins may always exist in the plasma membrane or be inserted when needed.
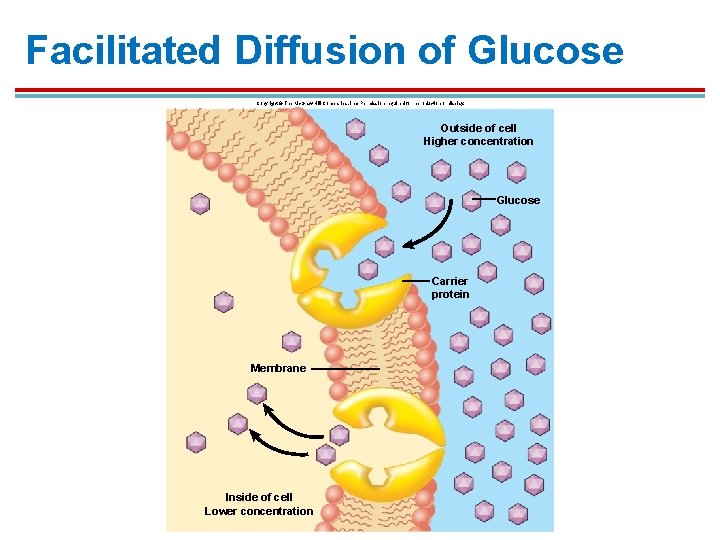
Facilitated Diffusion of Glucose Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Outside of cell Higher concentration Glucose Carrier protein Membrane Inside of cell Lower concentration
Facilitated Diffusion – Glucose 1. Transport carriers for glucose are designated GLUT followed by the number of the isoform 2. GLUT 1 – CNS 3. GLUT 2 – pancreatic beta cells & hepatocytes 4. GLUT 3 – neurons 5. GLUT 4 – adipose tissue & skeletal muscles; can be inserted into the plasma membrane of skeletal muscle when stimulated
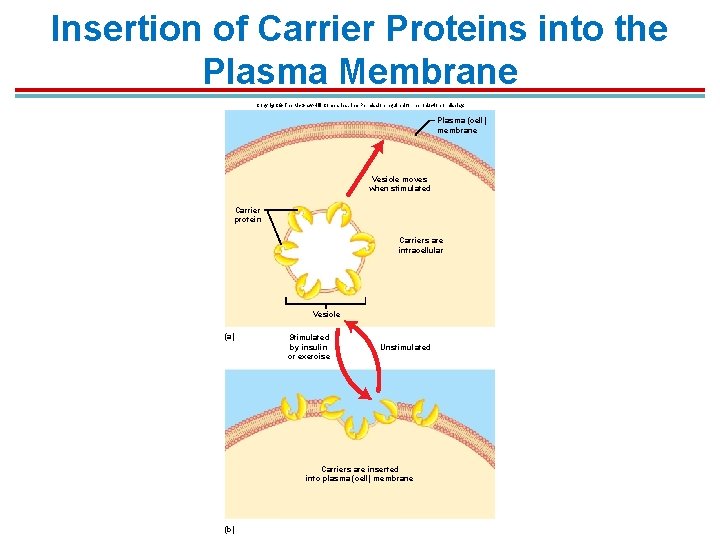
Insertion of Carrier Proteins into the Plasma Membrane Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Plasma (cell) membrane Vesicle moves when stimulated Carrier protein Carriers are intracellular Vesicle (a) Stimulated by insulin or exercise Unstimulated Carriers are inserted into plasma (cell) membrane (b)
Active Transport § Sometimes molecules must be moved from an area of low concentration to an area of high concentration (move uphill) § This requires the expenditure of ATP. § Often, these carrier-mediated proteins are called pumps.
Primary Active Transport 1. Occurs when the hydrolysis of ATP is directly responsible for the carrier protein function. 2. The transport protein is also an ATPase enzyme that will hydrolyze ATP 3. Pump (carrier-protein) is activated by phosphorylation using a Pi from ATP.
The Ca 2+ Pump 1. Located on all cells and in the endoplasmic reticulum of striated muscle cells 2. Removes Ca 2+ from the cytoplasm by pumping it into the extracellular fluid or cisternae of the ER 3. Creates a strong concentration gradient for rapid movement of Ca 2+ back into the cell 4. Ca 2+ aids in release of neurotransmitters in neurons and in muscle contraction
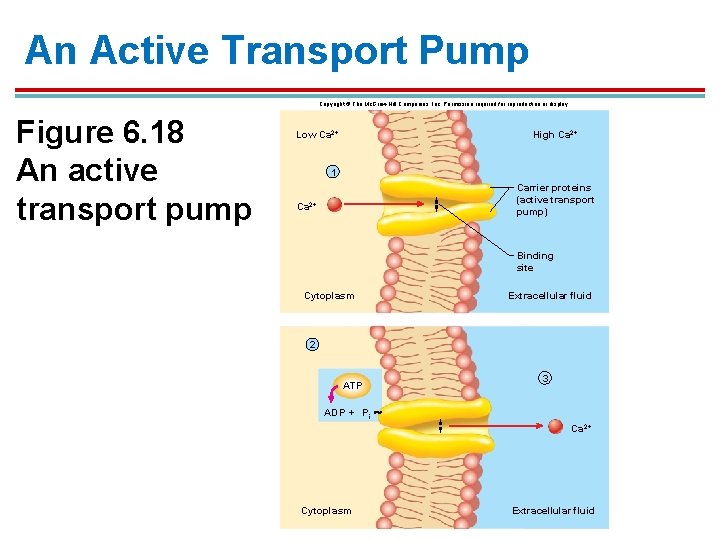
An Active Transport Pump Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Figure 6. 18 An active transport pump Low Ca 2+ High Ca 2+ 1 Carrier proteins (active transport pump) Ca 2+ Binding site Cytoplasm Extracellular fluid 2 ATP 3 ADP + Pi Ca 2+ Cytoplasm Extracellular fluid
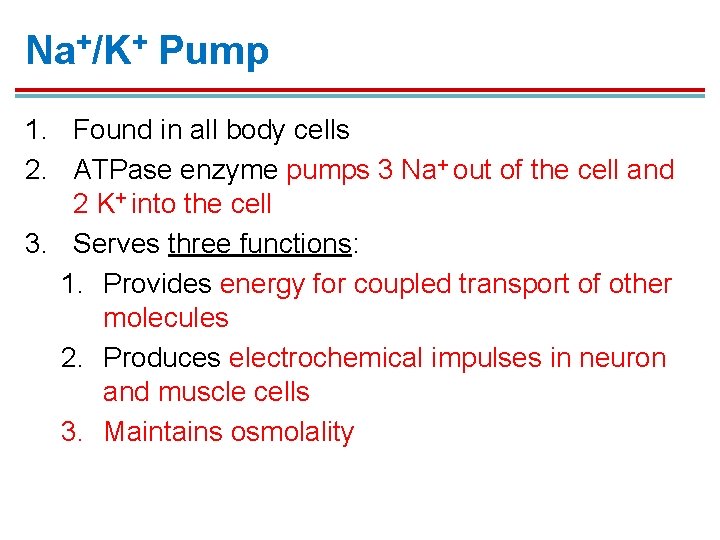
Na+/K+ Pump 1. Found in all body cells 2. ATPase enzyme pumps 3 Na+ out of the cell and 2 K+ into the cell 3. Serves three functions: 1. Provides energy for coupled transport of other molecules 2. Produces electrochemical impulses in neuron and muscle cells 3. Maintains osmolality
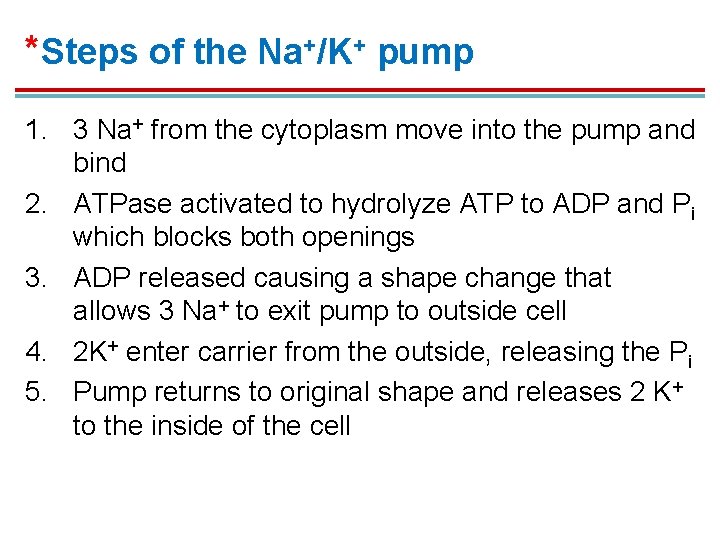
*Steps of the Na+/K+ pump 1. 3 Na+ from the cytoplasm move into the pump and bind 2. ATPase activated to hydrolyze ATP to ADP and Pi which blocks both openings 3. ADP released causing a shape change that allows 3 Na+ to exit pump to outside cell 4. 2 K+ enter carrier from the outside, releasing the Pi 5. Pump returns to original shape and releases 2 K+ to the inside of the cell
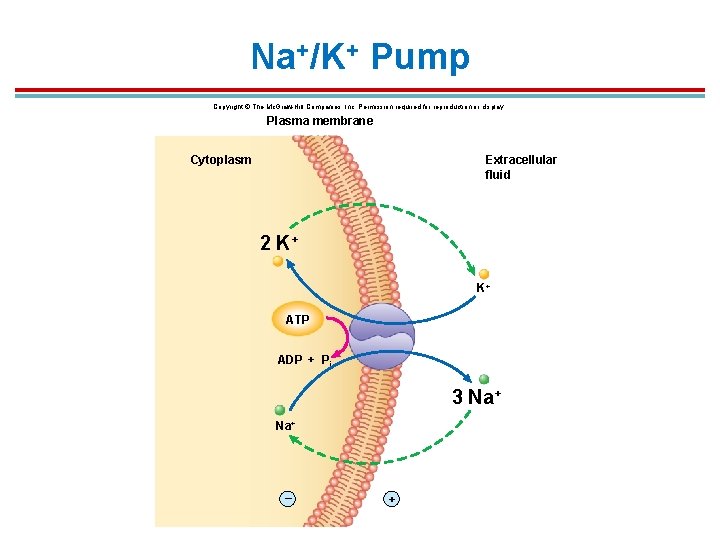
Na+/K+ Pump Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Plasma membrane Cytoplasm Extracellular fluid 2 K+ K+ ATP ADP + Pi 3 Na+ – +
Secondary Active Transport § § § Also called coupled transport The energy needed to move molecules across their concentration gradient is acquired by moving sodium back into the cell. Since the sodium was originally pumped out of the cell using ATP, this is considered active transport.
Secondary Active Transport § Cotransport or symport - the other molecule is moved with sodium. This is the common way to transport glucose § Countertransport or antiport - the other molecule is moved in the opposite direction from sodium. § An example is the uphill extrusion of Ca 2+ from a cell
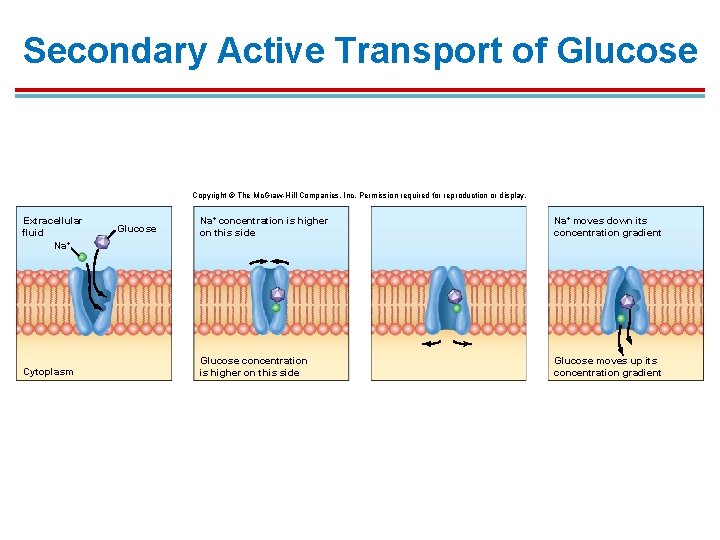
Secondary Active Transport of Glucose Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Extracellular fluid Na+ Cytoplasm Glucose Na+ concentration is higher on this side Na+ moves down its concentration gradient Glucose concentration is higher on this side Glucose moves up its concentration gradient
Transport Across Epithelial Membranes 1. Absorption – transport of digestive products across intestinal epithelium into the blood 2. Reabsorption – transport of molecules out of the urinary filtrate back into the blood 3. Involves transcellular transport: movement of molecules through the cytoplasm of the epithelial cells 4. May also involve paracellular transport: movement across the tiny gaps between cells
Transport Across Epithelial Membranes 1. Involves many different types of carrier- mediated proteins at both ends of the epithelial cells such as the Na+/K+ pump or the Na+/H+ pump
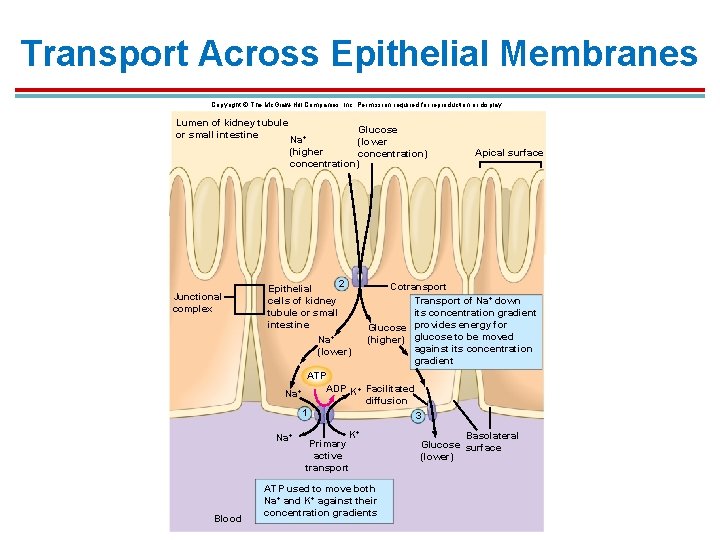
Transport Across Epithelial Membranes Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Lumen of kidney tubule or small intestine Junctional complex Glucose Na+ (lower (higher concentration) 2 Epithelial cells of kidney tubule or small intestine Na+ (lower) Apical surface Cotransport Transport of Na+ down its concentration gradient Glucose provides energy for (higher) glucose to be moved against its concentration gradient ATP ADP K+ Facilitated Na+ diffusion 1 3 Na+ Blood K+ Primary active transport ATP used to move both Na+ and K+ against their concentration gradients Basolateral Glucose surface (lower)
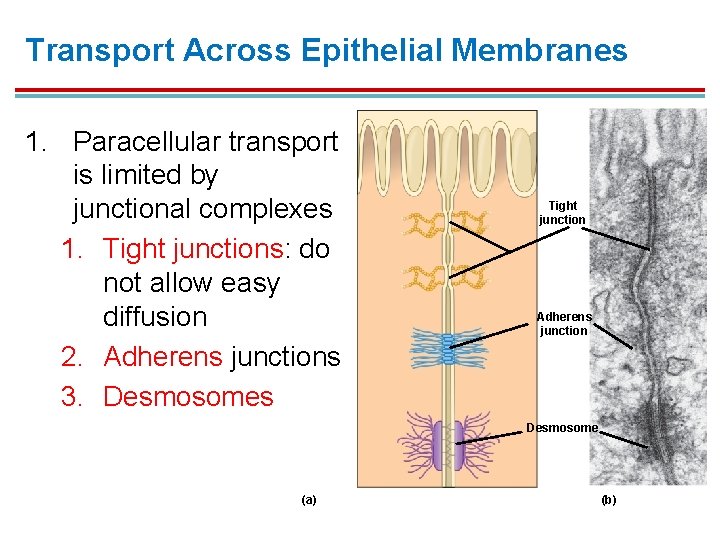
Transport Across Epithelial Membranes 1. Paracellular transport is limited by junctional complexes 1. Tight junctions: do not allow easy diffusion 2. Adherens junctions 3. Desmosomes Tight junction Adherens junction Desmosome (a) (b)
Bulk Transport § Large molecules such as proteins, hormones, and neurotransmitters are secreted via exocytosis. § Involves fusion of a vesicle with the plasma membrane § Requires ATP
Bulk Transport § Movement of large molecules such as cholesterol into the cell requires endocytosis. § Usually a transport protein interacts with plasma membrane proteins to trigger endocytosis.
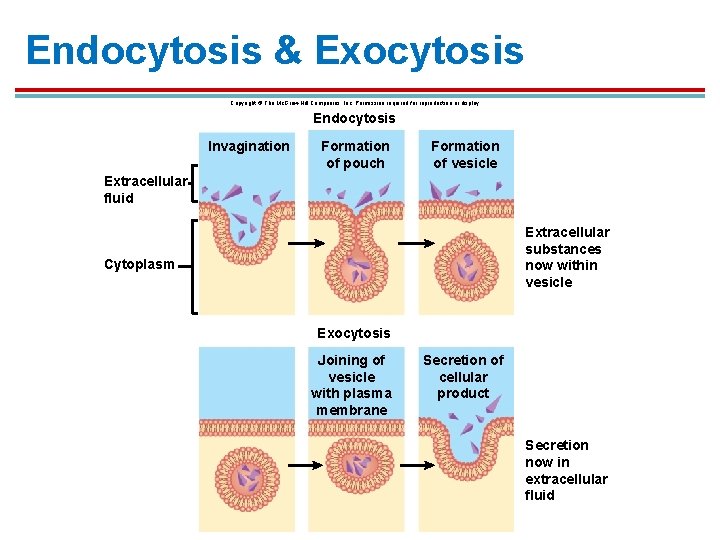
Endocytosis & Exocytosis Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Endocytosis Invagination Formation of pouch Formation of vesicle Extracellular fluid Extracellular substances now within vesicle Cytoplasm Exocytosis Joining of vesicle with plasma membrane Secretion of cellular product Secretion now in extracellular fluid

1. The Membrane Potential
Introduction § There is a difference in charge on each side of the plasma membrane due to: 1. Permeability of the membrane 2. Action of Na+/K+ pumps 3. Negatively charged molecules inside the cell § This difference in charge is called a potential difference, which makes the inside of the cell more negative compared to the outside.
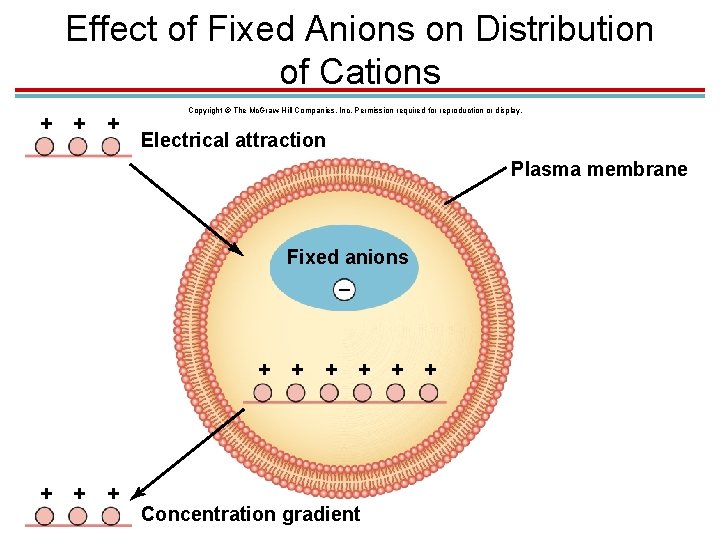
Effect of Fixed Anions on Distribution of Cations + + + Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Electrical attraction Plasma membrane Fixed anions – + + + + Concentration gradient + +

Membrane Potential: K+ § K+ accumulates at high concentrations in the cell because: 1. The Na+/K+ pumps actively bring in K+. 2. The membrane is very permeable to K+. 3. Negative anions inside the cell attract cations outside the cell. 4. Limited by strong concentration gradient. § The K+ concentration inside is 150 m. Eq/L and out is 5 m. Eq/L
Equilibrium Potentials § Even with all the K+ inside the cell, the negative molecules inside and all of the sodium outside, the cell is more negative inside compared to outside. § This potential difference can be measured as a voltage. § Because the membrane is so permeable to K+, this difference is often maintained by K+ concentration gradient

K+ Equilibrium § Addressing just K+, the electrical attraction would pull K+ into the cell until it reaches a point where the concentration gradient drawing K+ out matches this pull in. § K+ would reach an equilibrium, with more K+ inside than outside. § Normal cells have 150 m. M K+ inside and 5 m. M K+ outside.
K+ Equilibrium § The resulting potential difference measured in voltage would be the equilibrium potential (EK) for K+; measured at − 90 m. V. 1. This means the inside has a voltage 90 m. V lower than the outside. 2. This is the voltage needed to maintain 150 m. M K+ inside and 5 m. M K+ outside.
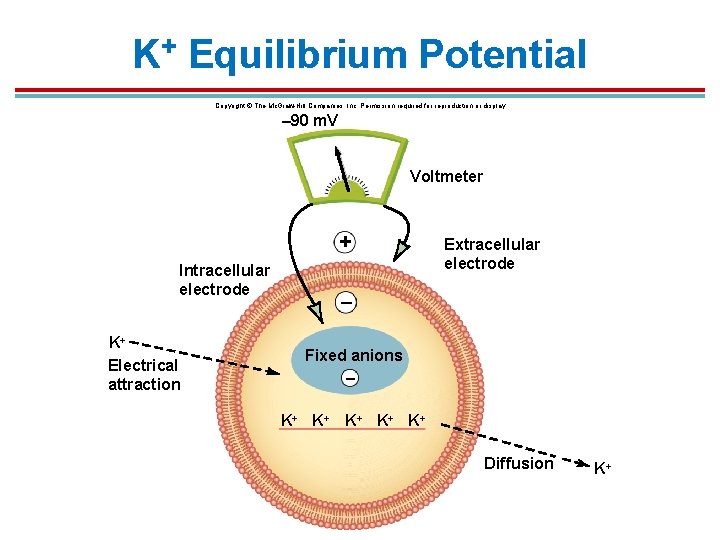
K+ Equilibrium Potential Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. – 90 m. V Voltmeter + Intracellular electrode K+ Electrical attraction Extracellular electrode – Fixed anions – K+ K+ K+ Diffusion K+

Na+ Equilibrium 1. Sodium is also an important ion for establishing membrane potential. 2. The concentration of sodium in a normal cell is 12 m. M inside and 145 m. M outside. 3. To keep so much sodium out, the inside would have to be positive to repel the sodium ions. 4. The equilibrium potential for sodium is +66 m. V. 5. The membrane is less permeable to Na+, so the actual membrane potential is closer to that of the more permeable K+.
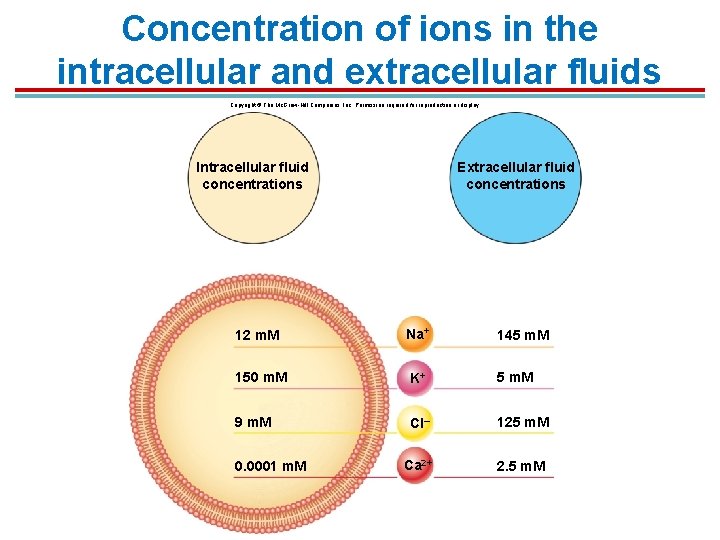
Concentration of ions in the intracellular and extracellular fluids Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Intracellular fluid concentrations Extracellular fluid concentrations 12 m. M Na+ 150 m. M K+ 5 m. M 9 m. M Cl– 125 m. M Ca 2+ 2. 5 m. M 0. 0001 m. M 145 m. M
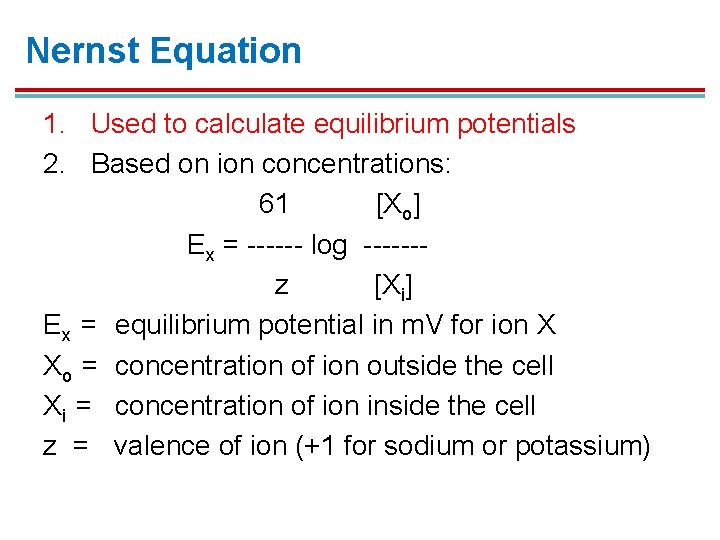
Nernst Equation 1. Used to calculate equilibrium potentials 2. Based on ion concentrations: 61 [Xo] Ex = ------ log ------z [Xi] Ex = equilibrium potential in m. V for ion X Xo = concentration of ion outside the cell Xi = concentration of ion inside the cell z = valence of ion (+1 for sodium or potassium)
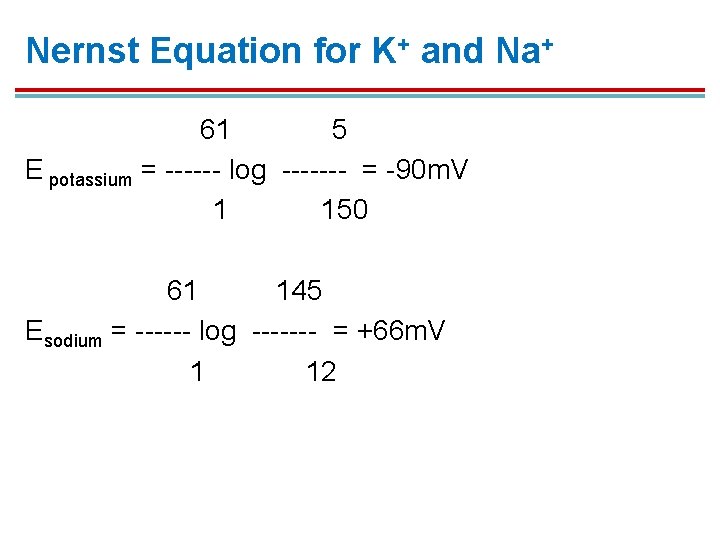
Nernst Equation for K+ and Na+ 61 5 E potassium = ------ log ------- = -90 m. V 1 150 61 145 Esodium = ------ log ------- = +66 m. V 1 12
Resting Membrane Potential § The value of a resting membrane potential of a cell, depends on: 1. Ratio of the concentrations of each ion on either side of the membrane 2. Specific permeability to each ion § K+, Na+, Ca 2+ and Cl− contribute to the resting potential.
Resting Membrane Potential § Causes of membrane potential change: § A change in the concentration of any ion inside or outside the cell will change the resting potential only to the extend that membrane is permeable to that ion. § A change in the membrane permeability to any given ion will change the membrane potential. § Key to how neurons work
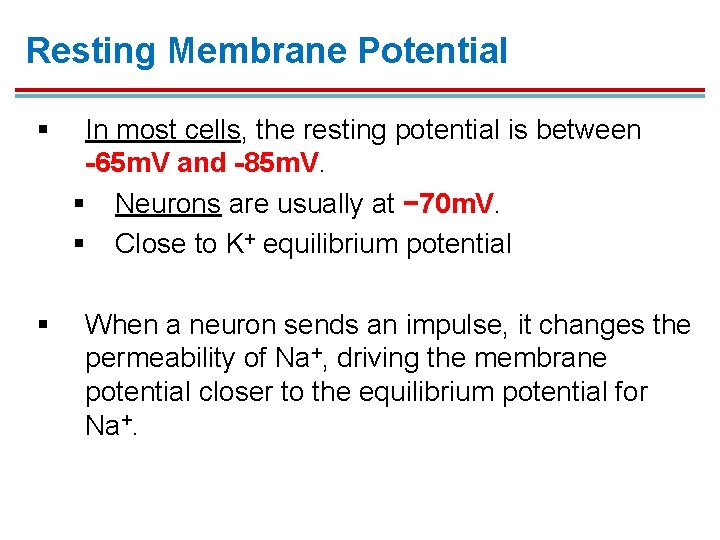
Resting Membrane Potential § § In most cells, the resting potential is between -65 m. V and -85 m. V. § Neurons are usually at − 70 m. V. § Close to K+ equilibrium potential When a neuron sends an impulse, it changes the permeability of Na+, driving the membrane potential closer to the equilibrium potential for Na+.
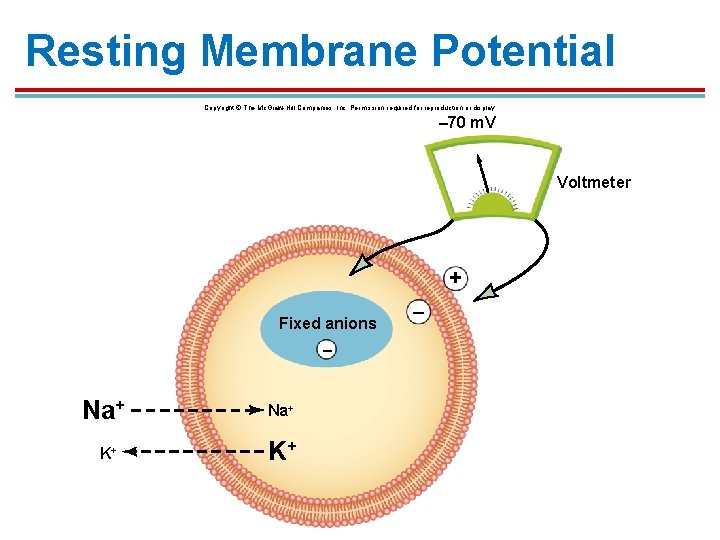
Resting Membrane Potential Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. – 70 m. V Voltmeter + Fixed anions – Na+ K+ –
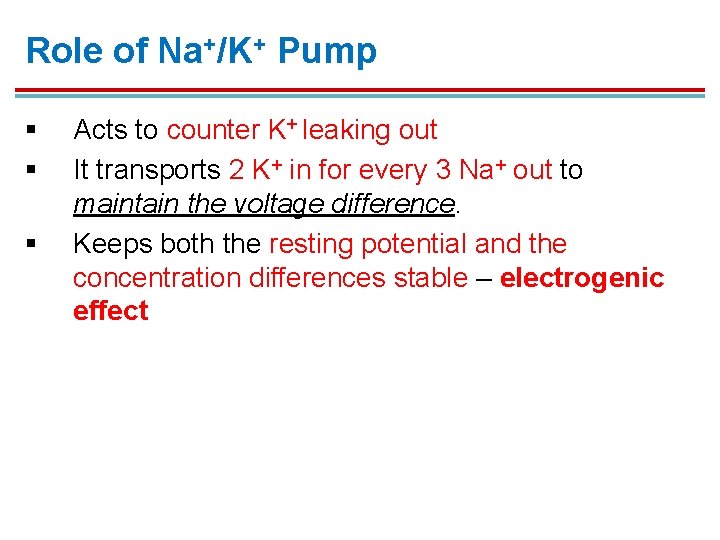
Role of Na+/K+ Pump § § § Acts to counter K+ leaking out It transports 2 K+ in for every 3 Na+ out to maintain the voltage difference. Keeps both the resting potential and the concentration differences stable – electrogenic effect
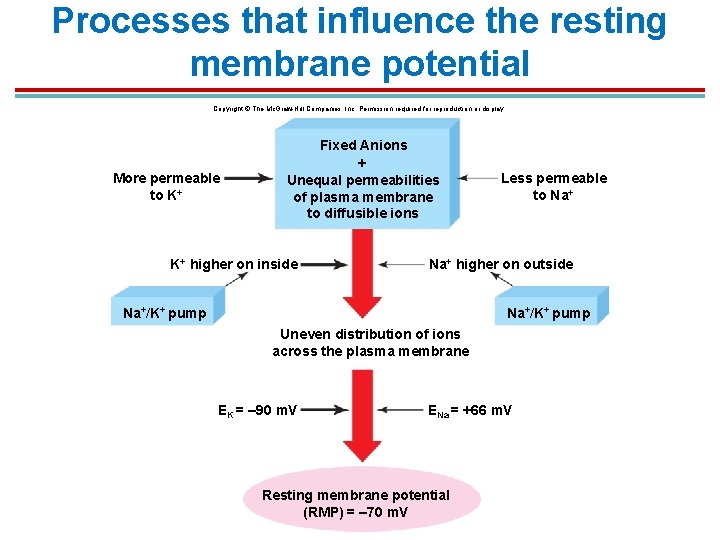
Processes that influence the resting membrane potential Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. More permeable to K+ Fixed Anions + Unequal permeabilities of plasma membrane to diffusible ions K+ higher on inside Less permeable to Na+ higher on outside Na+/K+ pump Uneven distribution of ions across the plasma membrane EK = – 90 m. V ENa = +66 m. V Resting membrane potential (RMP) = – 70 m. V

1. Cell Signaling
Introduction § § Cells communicate using chemical signals. Types: 1. Gap junctions: allow adjacent cells to pass ions and regulatory molecules through a channel between the cells 2. Paracrine signaling: Cells within an organ secrete molecules that diffuse across the extracellular space to nearby target cells; often called local signaling
Cell Signaling types 1. Synaptic signaling: involves neurons secreting neurotransmitters across a synapse to target cells 2. Endocrine signaling: involves glands that secrete hormones into the bloodstream; these can reach multiple target cells
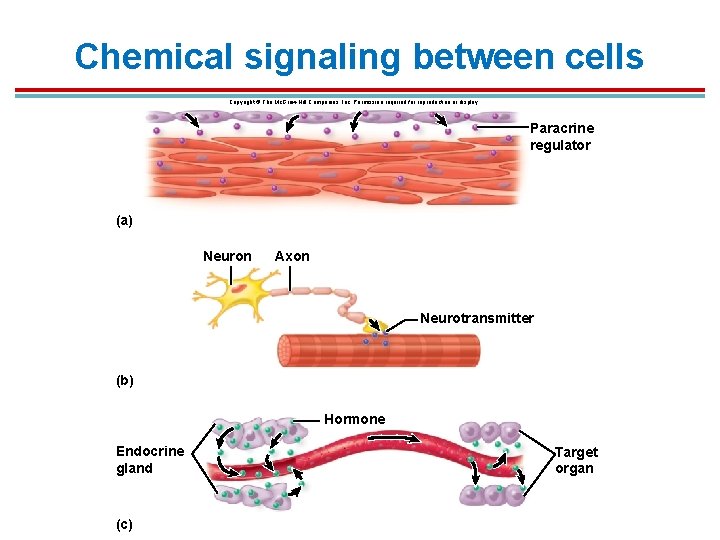
Chemical signaling between cells Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Paracrine regulator (a) Neuron Axon Neurotransmitter (b) Hormone Endocrine gland (c) Target organ
Receptor Proteins § A target cell receives a signal because it has receptor proteins specific to it on the plasma membrane or inside the cell. 1. Nonpolar signal molecules such as steroid hormones, thyroid hormone, and nitric oxide gas can penetrate the plasma membrane and interact with receptors inside the cell. 2. Large, polar signal molecules such as epinephrine, acetylcholine, and insulin bond to receptors on the plasma membrane and activates second messengers
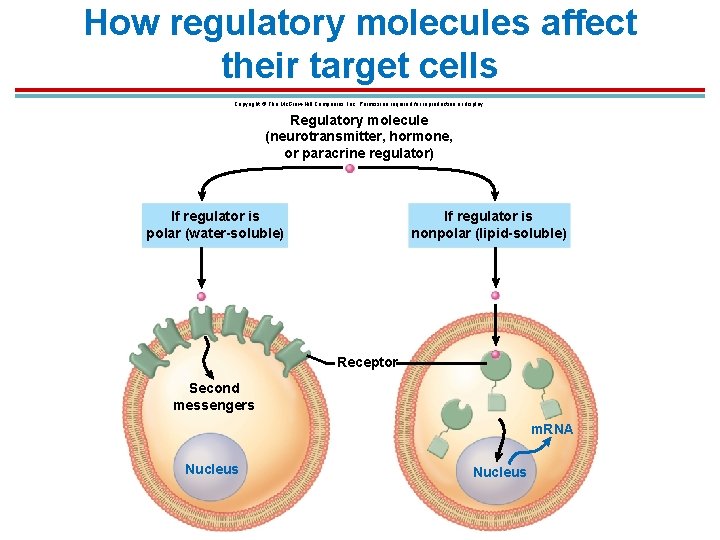
How regulatory molecules affect their target cells Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Regulatory molecule (neurotransmitter, hormone, or paracrine regulator) If regulator is polar (water-soluble) If regulator is nonpolar (lipid-soluble) Receptor Second messengers m. RNA Nucleus
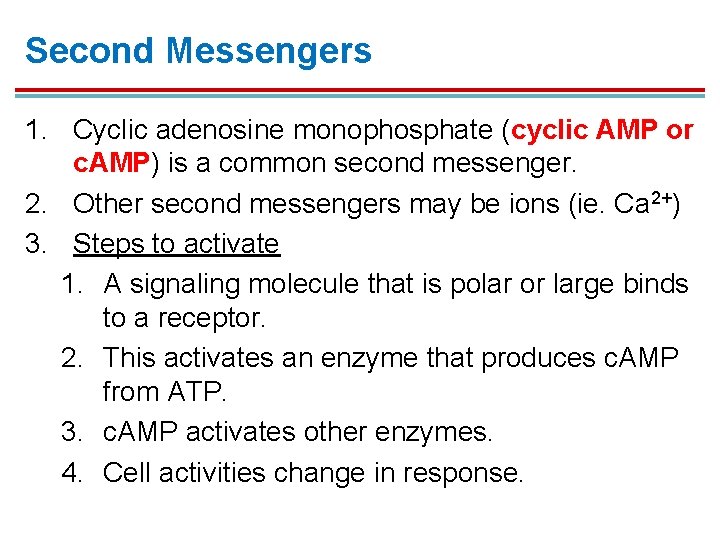
Second Messengers 1. Cyclic adenosine monophosphate (cyclic AMP or c. AMP) is a common second messenger. 2. Other second messengers may be ions (ie. Ca 2+) 3. Steps to activate 1. A signaling molecule that is polar or large binds to a receptor. 2. This activates an enzyme that produces c. AMP from ATP. 3. c. AMP activates other enzymes. 4. Cell activities change in response.
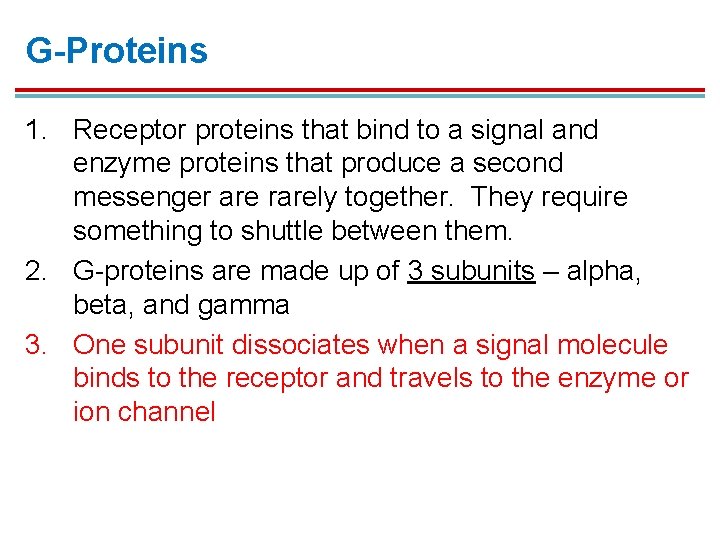
G-Proteins 1. Receptor proteins that bind to a signal and enzyme proteins that produce a second messenger are rarely together. They require something to shuttle between them. 2. G-proteins are made up of 3 subunits – alpha, beta, and gamma 3. One subunit dissociates when a signal molecule binds to the receptor and travels to the enzyme or ion channel
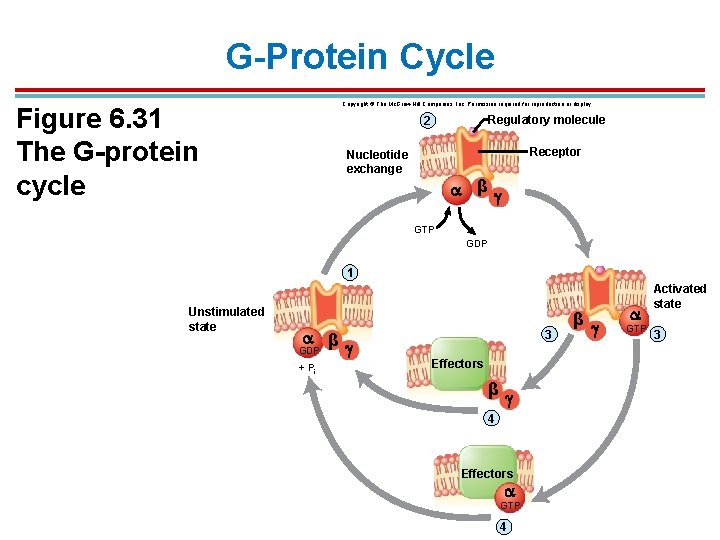
G-Protein Cycle Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Figure 6. 31 The G-protein cycle Regulatory molecule 2 Receptor Nucleotide exchange β GTP GDP 1 Unstimulated state β GDP + Pi 3 Effectors β 4 Effectors GTP 4 β GTP Activated state 3
- Slides: 94