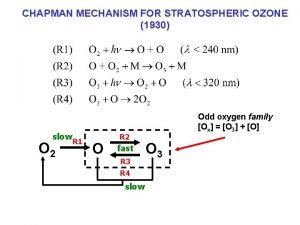
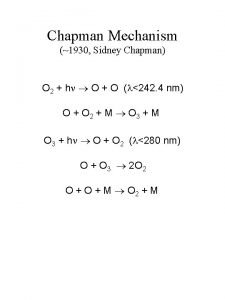
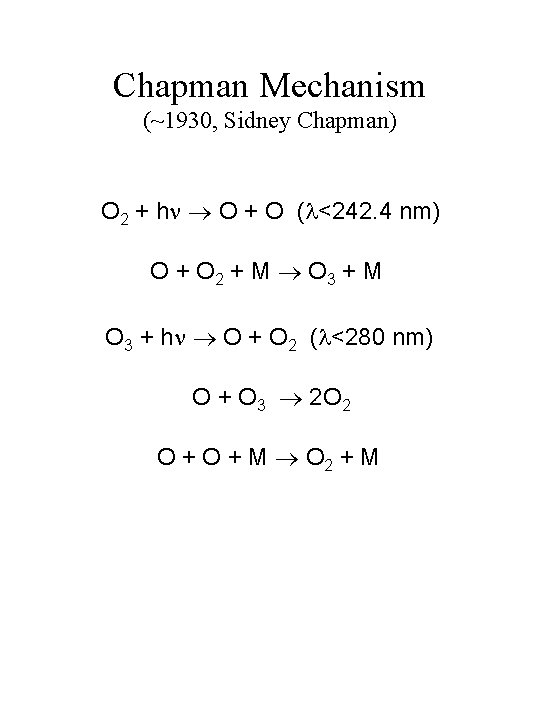
Chapman Mechanism 1930 Sidney Chapman O 2 h







![In lower stratosphere (~15 -25 km), [O] is relatively low: UV-C absorbed by ozone. In lower stratosphere (~15 -25 km), [O] is relatively low: UV-C absorbed by ozone.](https://slidetodoc.com/presentation_image_h/114b230060b4aa8d11926696f0dd31dd/image-13.jpg)






- Slides: 22

Chapman Mechanism (~1930, Sidney Chapman) O 2 + h O + O ( <242. 4 nm) O + O 2 + M O 3 + M O 3 + h O + O 2 ( <280 nm) O + O 3 2 O 2 O + M O 2 + M







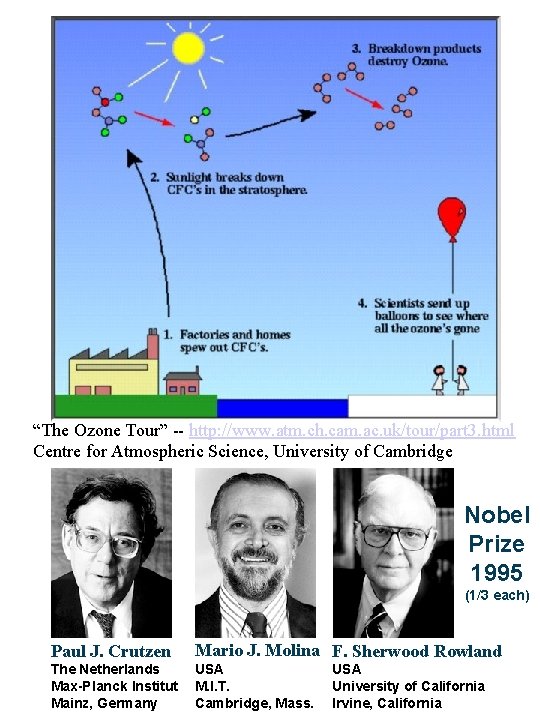
http: //www. epa. gov/indicators/roe/html/roe. Air. Info. htm United States Environmental Protection Agency EPA Report on the Environment (ROE) http: //www. atm. ch. cam. ac. uk/tour/part 2. html “The Ozone Tour” Centre for Atmospheric Science, University of Cambridge


“The Ozone Tour” -- http: //www. atm. ch. cam. ac. uk/tour/part 3. html Centre for Atmospheric Science, University of Cambridge Nobel Prize 1995 (1/3 each) Paul J. Crutzen Mario J. Molina F. Sherwood Rowland The Netherlands Max-Planck Institut Mainz, Germany USA M. I. T. Cambridge, Mass. USA University of California Irvine, California


Catalytic Decomposition of Ozone X + O 3 XO + O 2 XO + O X + O 2 _______________ __ O 3 + O 2 O 2 X = HOx ( H, OH, HOO ) NOx (NO , NO 2) Cl. Ox ( Cl, Cl. O ) N 2 O from troposphere: N 2 O + O* 2 NO in middle & upper stratosphere NO + O 3 NO 2 + O 2 NO 2 + O NO + O 2 _______________ __ O 3 + O 2 O 2 Above 45 km, OH dominates, from: O* + H 2 O OH + OH and O* + CH 4 OH + CH 3 HO + O 3 HOO + O 2 HOO + O HO + O 2 ________________ O 3 + O 2 O 2
![In lower stratosphere 15 25 km O is relatively low UVC absorbed by ozone In lower stratosphere (~15 -25 km), [O] is relatively low: UV-C absorbed by ozone.](https://slidetodoc.com/presentation_image_h/114b230060b4aa8d11926696f0dd31dd/image-13.jpg)
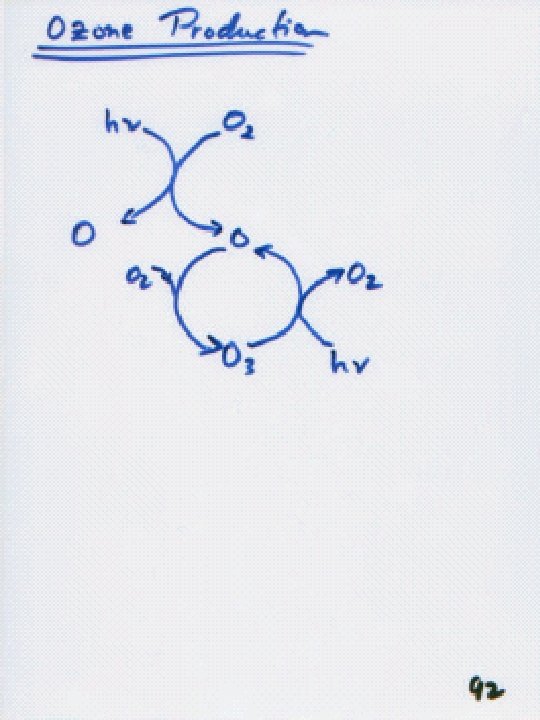
In lower stratosphere (~15 -25 km), [O] is relatively low: UV-C absorbed by ozone. [O 2] is high (so most O quickly reacts with it). Therefore, the dominant ozone loss mechanism is: X + O 3 XO + O 2 X + O 3 X O + O 2 XO + X O X + O 2 ________________ 2 O 3 3 O 2 Reaction goes by: XO + X O [XOOX ] X + O 2 Rate of O 3 production depends on [O 2], [O 3], h (UV-C) Destruction is more complex, but depends on [X], UV-B. If something changes, generally [O 3] increases or decreases until it reaches a steady state. Self-healing: [O 3] , UV-C , more O 3 forms below. Next: Atomic Cl and Br as X.

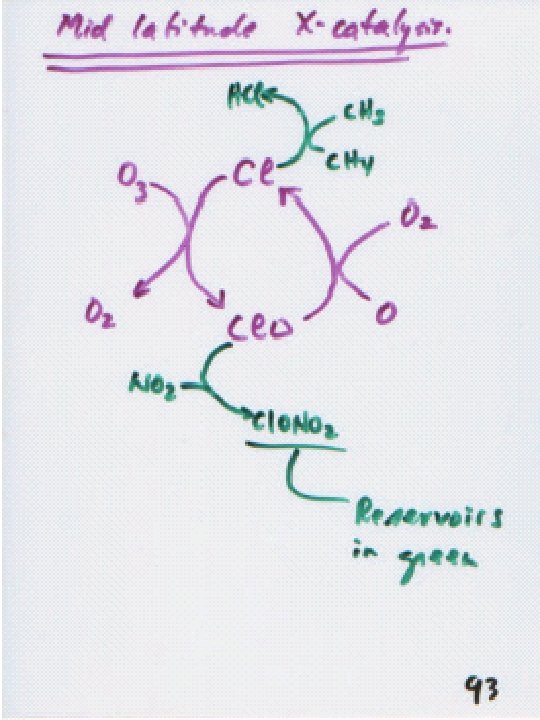
Atomic Cl and Br as X: Cl can destroy tens of thousands of O 3 molecules each, but is mainly in inactive forms (HCl, Cl. ONO 2) in stratosphere. Cl. O + NO 2 Cl. ONO 2 Cl + CH 4 HCl + CH 3 does not operate as an X catalyst, since it combines with O 2 to give CO 2.



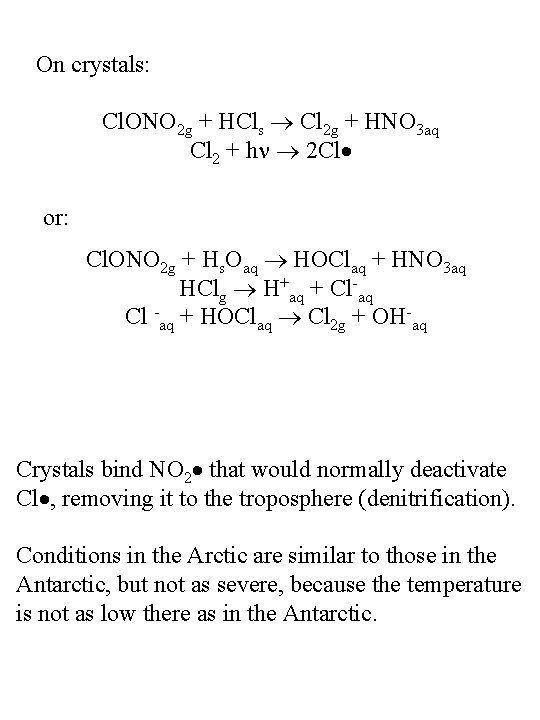
On crystals: Cl. ONO 2 g + HCls Cl 2 g + HNO 3 aq Cl 2 + h 2 Cl or: Cl. ONO 2 g + Hs. Oaq HOClaq + HNO 3 aq HClg H+aq + Cl-aq Cl -aq + HOClaq Cl 2 g + OH-aq Crystals bind NO 2 that would normally deactivate Cl , removing it to the troposphere (denitrification). Conditions in the Arctic are similar to those in the Antarctic, but not as severe, because the temperature is not as low there as in the Antarctic.





 Chapman
Chapman Chapman mechanism
Chapman mechanism Slovenski ekspresionizem
Slovenski ekspresionizem Nguyễn ái quốc 1930
Nguyễn ái quốc 1930 Sebastian bredal
Sebastian bredal Sale of goods act 1930 introduction
Sale of goods act 1930 introduction Who attended all three round table conference
Who attended all three round table conference 2010-1930
2010-1930 Kviz o fudbalu
Kviz o fudbalu Moralismo pedagógico de los estados unidos
Moralismo pedagógico de los estados unidos đảng cộng sản việt nam 1930
đảng cộng sản việt nam 1930 Funding loan
Funding loan 1930-1939 fashion
1930-1939 fashion Moda anilor 1930
Moda anilor 1930 Guam and puerto rico
Guam and puerto rico The place where in futsal created in 1930 was in
The place where in futsal created in 1930 was in 1930 computers
1930 computers History of the atom
History of the atom Castrovalva escher
Castrovalva escher 1930 pop culture
1930 pop culture Becker 1930
Becker 1930 2007-1930
2007-1930 Feminist naacp leader 1930-1950
Feminist naacp leader 1930-1950