Chap 5 part II Alkenes and Alkynes II















- Slides: 44

Chap. 5 part II Alkenes and Alkynes II: Addition Reactions Solomons, Chapter 8

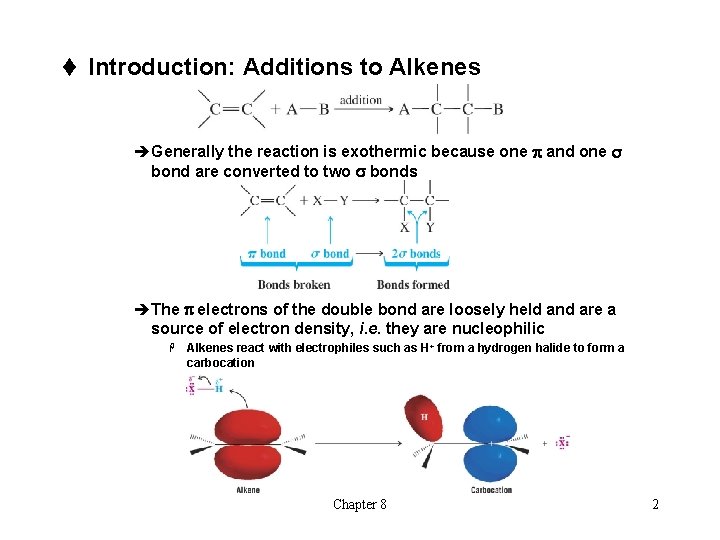
t Introduction: Additions to Alkenes èGenerally the reaction is exothermic because one p and one s bond are converted to two s bonds èThe p electrons of the double bond are loosely held and are a source of electron density, i. e. they are nucleophilic H Alkenes react with electrophiles such as H+ from a hydrogen halide to form a carbocation Chapter 8 2

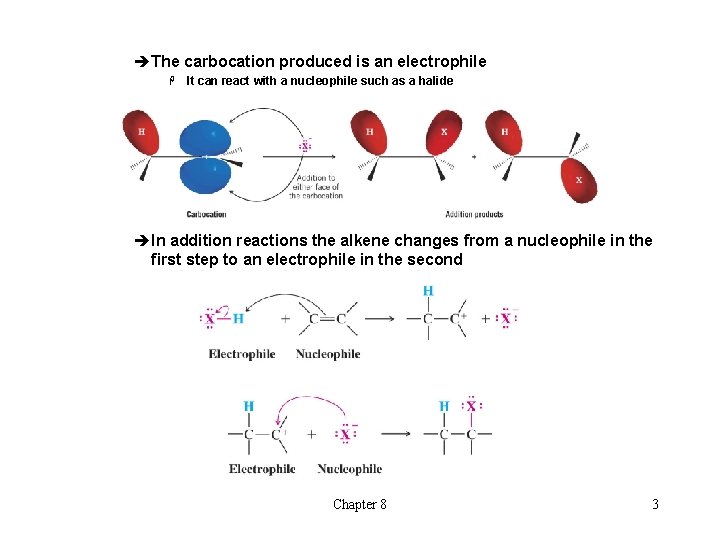
èThe carbocation produced is an electrophile H It can react with a nucleophile such as a halide » Insert top scheme pg 331 èIn addition reactions the alkene changes from a nucleophile in the first step to an electrophile in the second Chapter 8 3

Addition of Hydrogen Halides to Alkenes Chapter 8 4

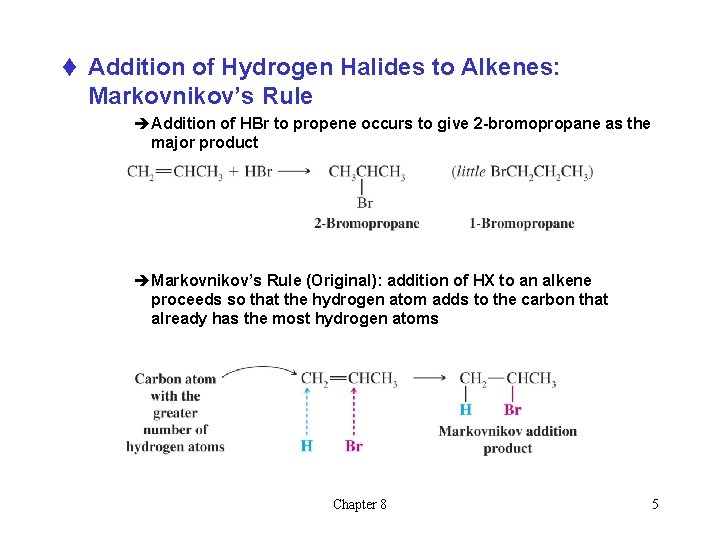
t Addition of Hydrogen Halides to Alkenes: Markovnikov’s Rule èAddition of HBr to propene occurs to give 2 -bromopropane as the major product èMarkovnikov’s Rule (Original): addition of HX to an alkene proceeds so that the hydrogen atom adds to the carbon that already has the most hydrogen atoms Chapter 8 5

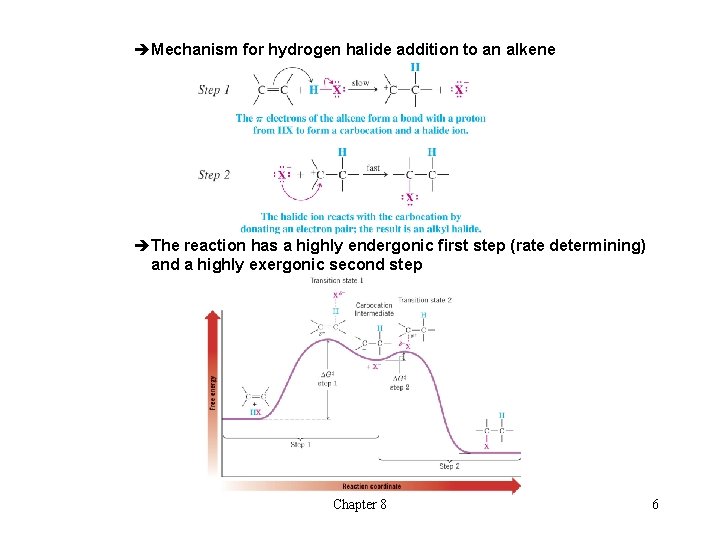
èMechanism for hydrogen halide addition to an alkene èThe reaction has a highly endergonic first step (rate determining) and a highly exergonic second step Chapter 8 6

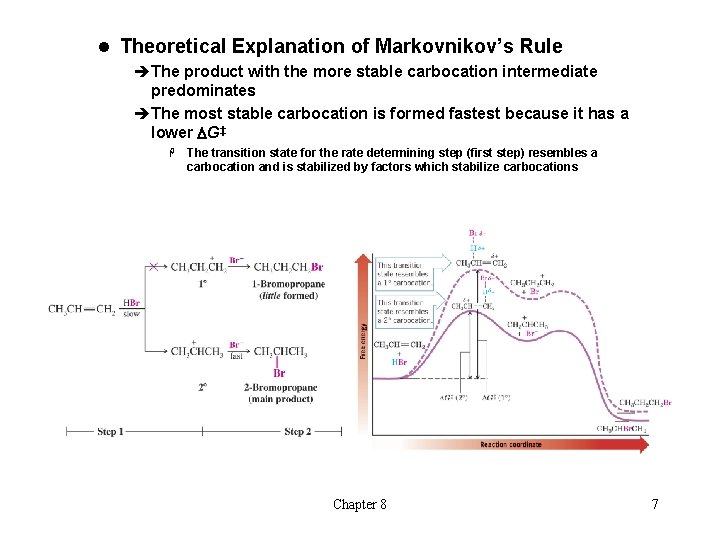
l Theoretical Explanation of Markovnikov’s Rule èThe product with the more stable carbocation intermediate predominates èThe most stable carbocation is formed fastest because it has a lower DG‡ H The transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations Chapter 8 7

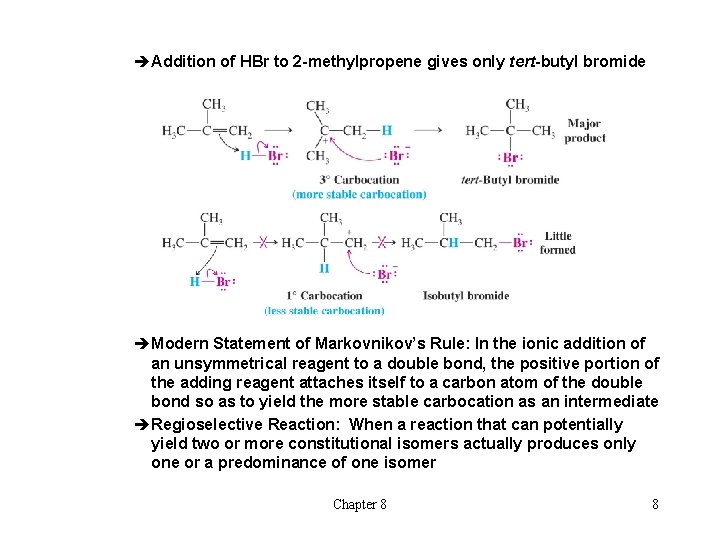
èAddition of HBr to 2 -methylpropene gives only tert-butyl bromide èModern Statement of Markovnikov’s Rule: In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate èRegioselective Reaction: When a reaction that can potentially yield two or more constitutional isomers actually produces only one or a predominance of one isomer Chapter 8 8

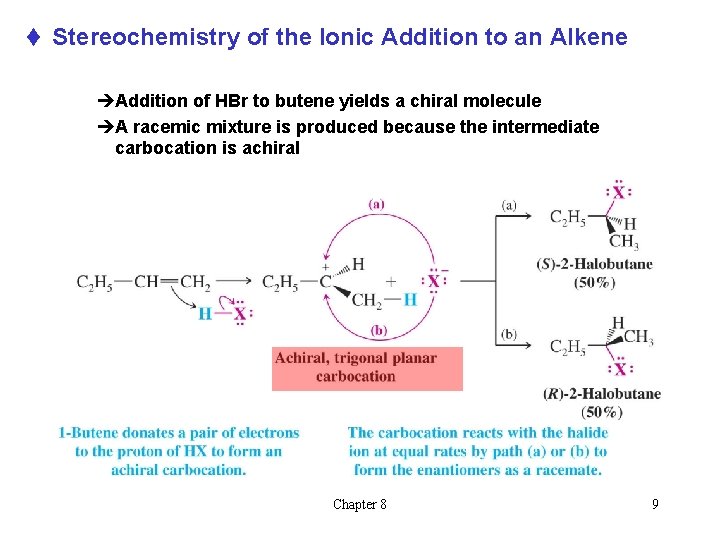
t Stereochemistry of the Ionic Addition to an Alkene èAddition of HBr to butene yields a chiral molecule èA racemic mixture is produced because the intermediate carbocation is achiral Chapter 8 9

Addition of Sulfuric Acid to Alkenes Chapter 8 10

t Addition of Sulfuric Acid to Alkenes èAddition of concentrated sulfuric acid to alkenes leads to alkyl hydrogen sulfates which are soluble in the acid H The addition follows Markovnikov’s rule èThe sulfate can be hydrolyzed by heating with water H The net result is Markovnikov addition of water to an alkene Chapter 8 11

Addition of Water to Alkenes Chapter 8 12

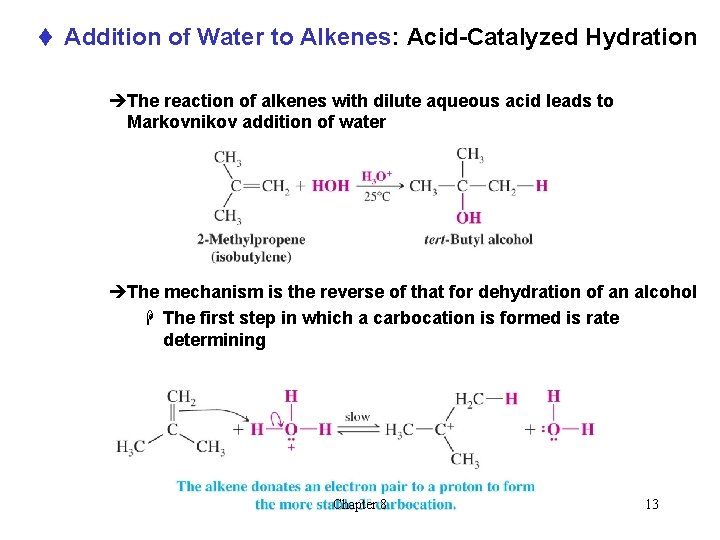
t Addition of Water to Alkenes: Acid-Catalyzed Hydration èThe reaction of alkenes with dilute aqueous acid leads to Markovnikov addition of water èThe mechanism is the reverse of that for dehydration of an alcohol H The first step in which a carbocation is formed is rate determining Chapter 8 13

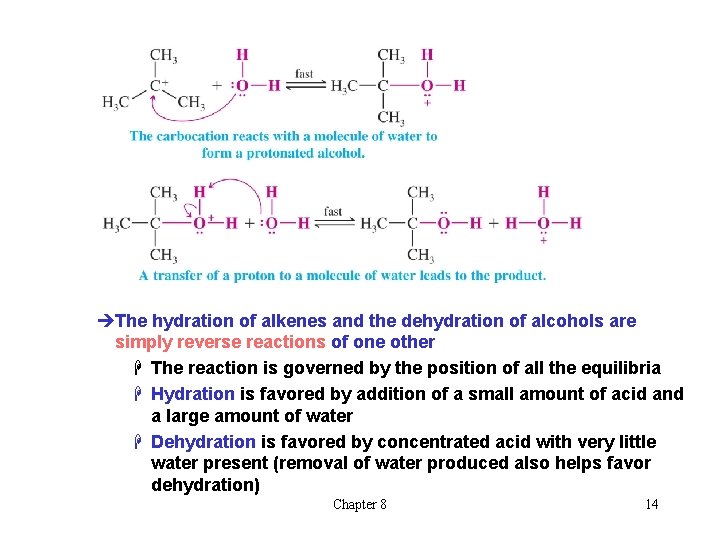
èThe hydration of alkenes and the dehydration of alcohols are simply reverse reactions of one other H The reaction is governed by the position of all the equilibria H Hydration is favored by addition of a small amount of acid and a large amount of water H Dehydration is favored by concentrated acid with very little water present (removal of water produced also helps favor dehydration) Chapter 8 14

Carbocation rearrangements can occur Chapter 8 15

Hydroboration-Oxidation Chapter 8 16

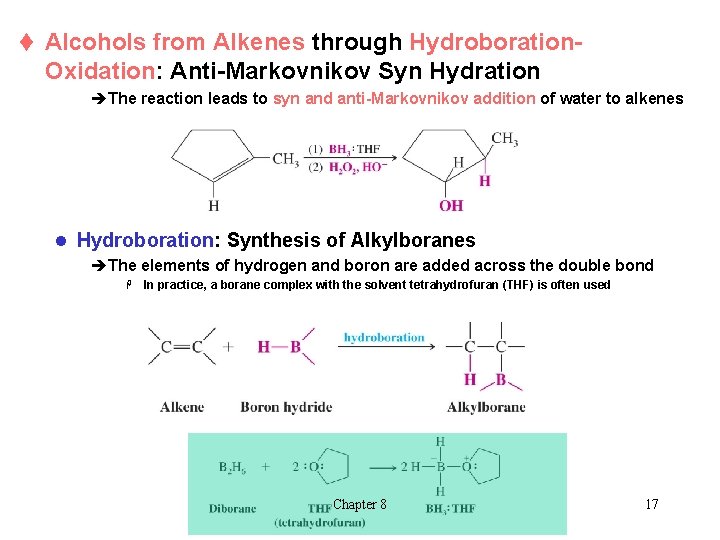
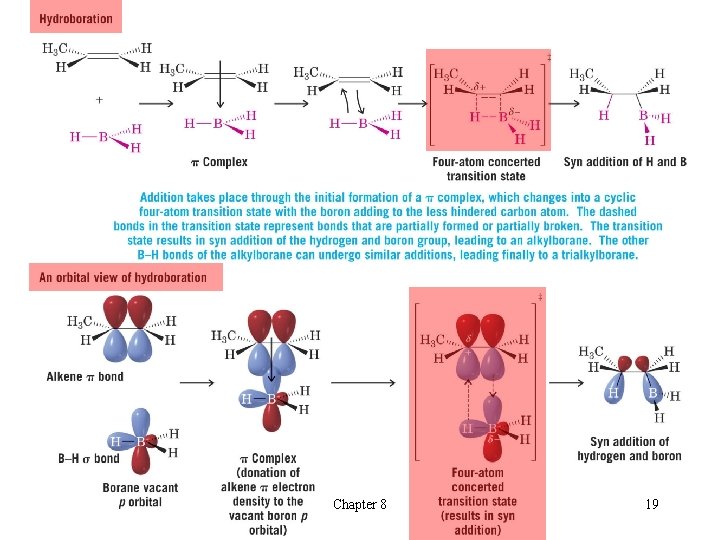
t Alcohols from Alkenes through Hydroboration- Oxidation: Anti-Markovnikov Syn Hydration èThe reaction leads to syn and anti-Markovnikov addition of water to alkenes l Hydroboration: Synthesis of Alkylboranes èThe elements of hydrogen and boron are added across the double bond H In practice, a borane complex with the solvent tetrahydrofuran (THF) is often used Chapter 8 17

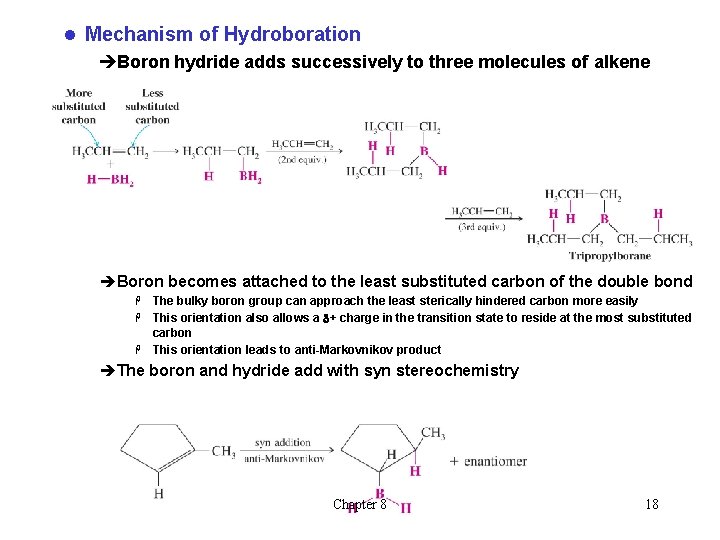
l Mechanism of Hydroboration èBoron hydride adds successively to three molecules of alkene èBoron becomes attached to the least substituted carbon of the double bond The bulky boron group can approach the least sterically hindered carbon more easily H This orientation also allows a d+ charge in the transition state to reside at the most substituted carbon H This orientation leads to anti-Markovnikov product H èThe boron and hydride add with syn stereochemistry Chapter 8 18

Chapter 8 19

l Oxidation and Hydrolysis of Alkylboranes èOxidation and hydrolysis to the alcohol takes place with retention of stereochemistry at the carbon bonded to boron Chapter 8 20

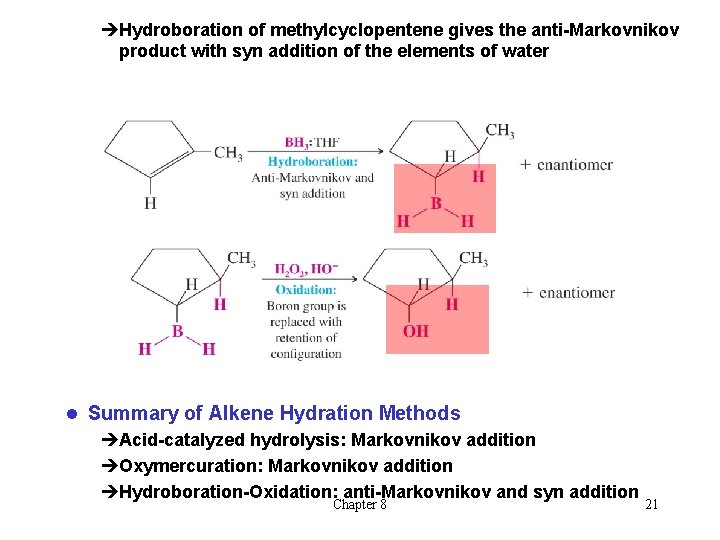
èHydroboration of methylcyclopentene gives the anti-Markovnikov product with syn addition of the elements of water l Summary of Alkene Hydration Methods èAcid-catalyzed hydrolysis: Markovnikov addition èOxymercuration: Markovnikov addition èHydroboration-Oxidation: anti-Markovnikov and syn addition Chapter 8 21

Addition of Bromine and Chlorine to Alkenes Chapter 8 22

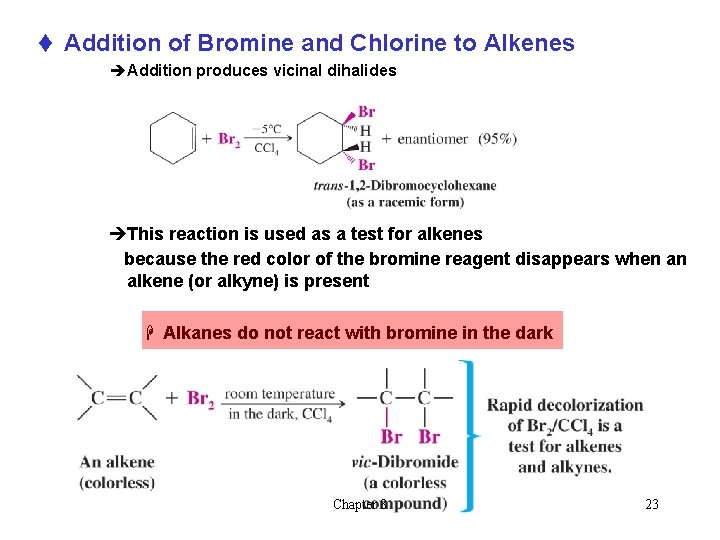
t Addition of Bromine and Chlorine to Alkenes èAddition produces vicinal dihalides èThis reaction is used as a test for alkenes because the red color of the bromine reagent disappears when an alkene (or alkyne) is present H Alkanes do not react with bromine in the dark Chapter 8 23

l Mechanism of Halogen Addition èA bromonium ion intermediate results instead of the carbocation seen in other addition reactions Chapter 8 24

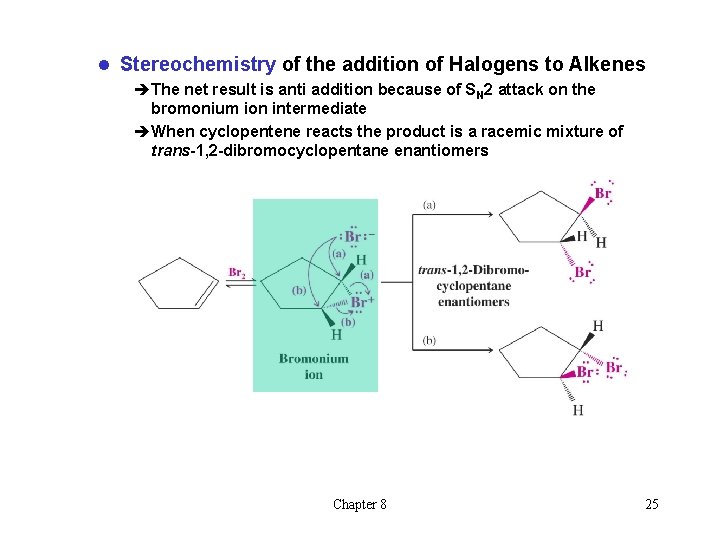
l Stereochemistry of the addition of Halogens to Alkenes èThe net result is anti addition because of SN 2 attack on the bromonium ion intermediate èWhen cyclopentene reacts the product is a racemic mixture of trans-1, 2 -dibromocyclopentane enantiomers Chapter 8 25

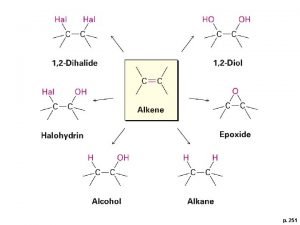
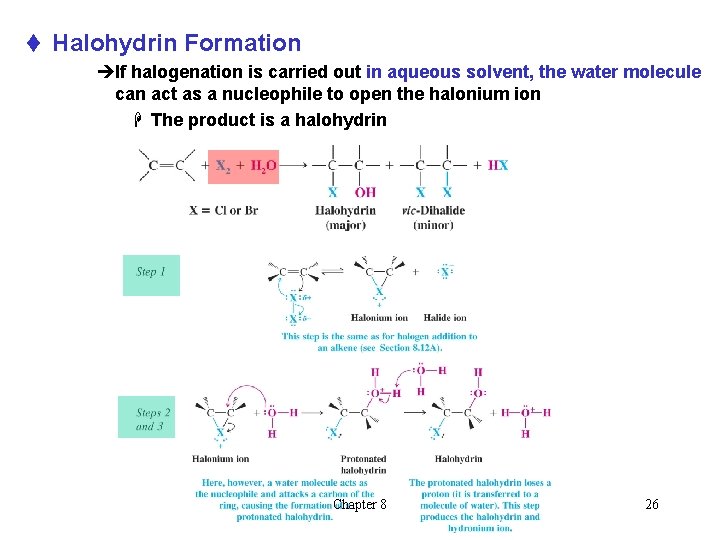
t Halohydrin Formation èIf halogenation is carried out in aqueous solvent, the water molecule can act as a nucleophile to open the halonium ion H The product is a halohydrin Chapter 8 26

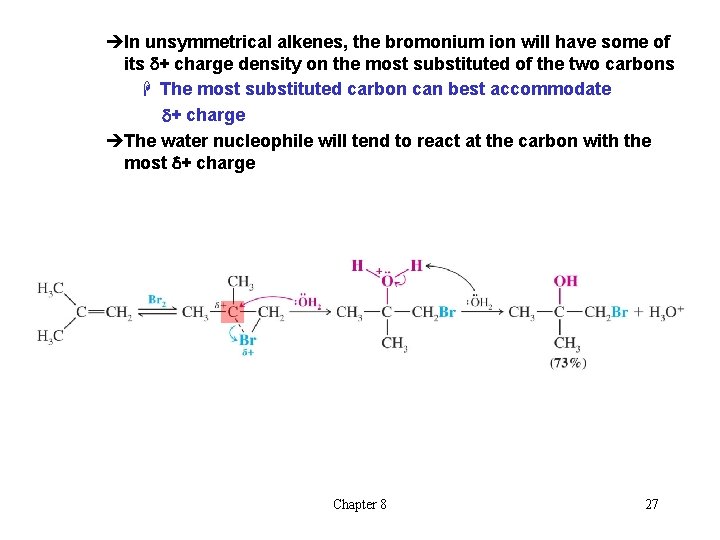
èIn unsymmetrical alkenes, the bromonium ion will have some of its d+ charge density on the most substituted of the two carbons H The most substituted carbon can best accommodate d+ charge èThe water nucleophile will tend to react at the carbon with the most d+ charge Chapter 8 27

Divalent Carbon Compounds: Carbenes Chapter 8 28

t Divalent Carbon Compounds: Carbenes èCarbenes have divalent but neutral carbons with a lone pair of electrons H Carbenes are highly reactive l Structure and Reaction of Methylene èMethylene can be made by heat or light initiated decomposition of diazomethane H Loss of a molecule of the stable gas nitrogen drives this reaction èMethylene reacts with alkenes to form cyclopropanes Chapter 8 29

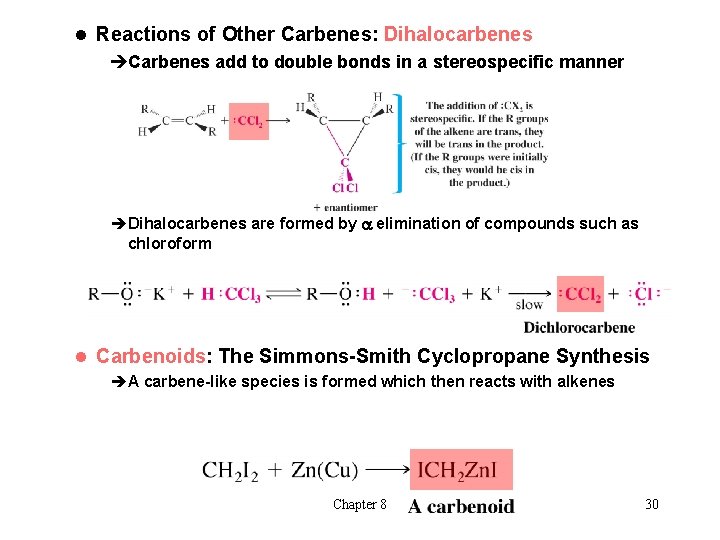
l Reactions of Other Carbenes: Dihalocarbenes èCarbenes add to double bonds in a stereospecific manner èDihalocarbenes are formed by a elimination of compounds such as chloroform l Carbenoids: The Simmons-Smith Cyclopropane Synthesis èA carbene-like species is formed which then reacts with alkenes Chapter 8 30

Oxidations of Alkenes Os. O 4酸化 KMn. O 4酸化 O 3酸化 Chapter 8 31

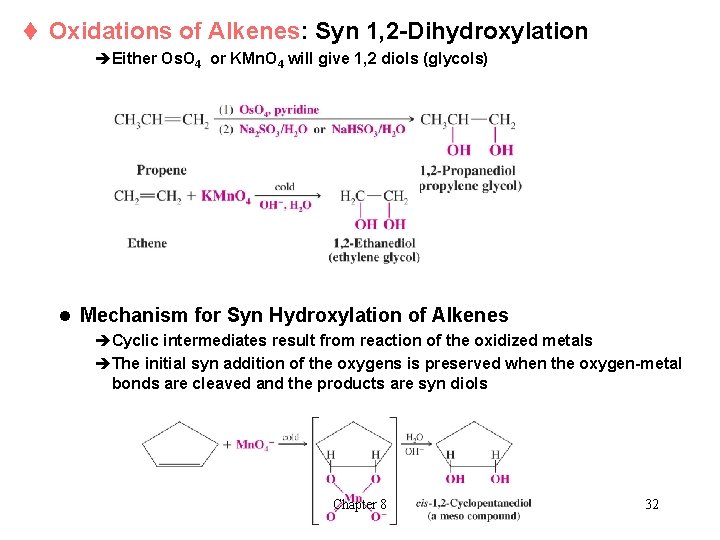
t Oxidations of Alkenes: Syn 1, 2 -Dihydroxylation èEither Os. O 4 or KMn. O 4 will give 1, 2 diols (glycols) l Mechanism for Syn Hydroxylation of Alkenes èCyclic intermediates result from reaction of the oxidized metals èThe initial syn addition of the oxygens is preserved when the oxygen-metal bonds are cleaved and the products are syn diols Chapter 8 32

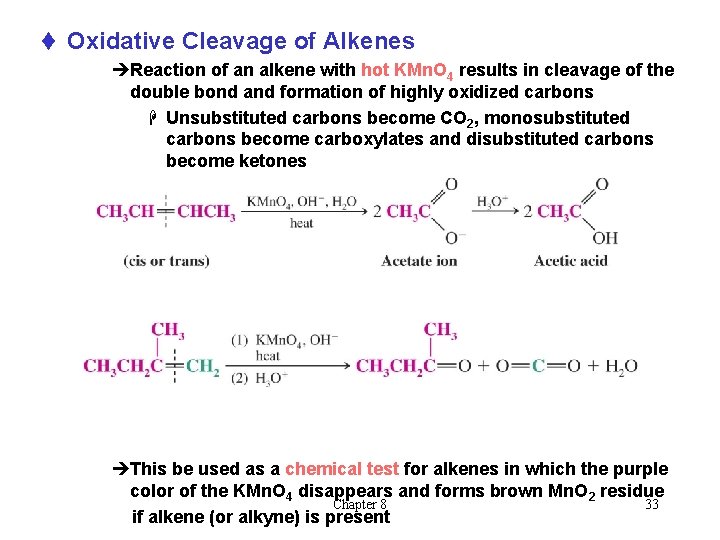
t Oxidative Cleavage of Alkenes èReaction of an alkene with hot KMn. O 4 results in cleavage of the double bond and formation of highly oxidized carbons H Unsubstituted carbons become CO 2, monosubstituted carbons become carboxylates and disubstituted carbons become ketones èThis be used as a chemical test for alkenes in which the purple color of the KMn. O 4 disappears and forms brown Mn. O 2 residue Chapter 8 33 if alkene (or alkyne) is present

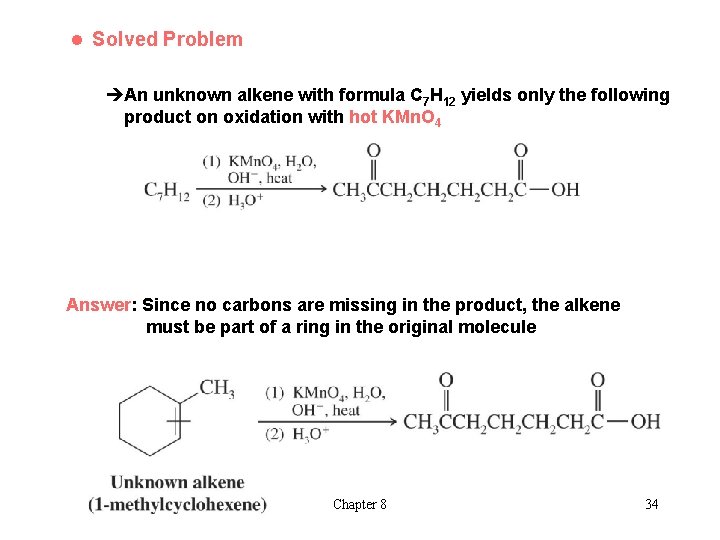
l Solved Problem èAn unknown alkene with formula C 7 H 12 yields only the following product on oxidation with hot KMn. O 4 Answer: Since no carbons are missing in the product, the alkene must be part of a ring in the original molecule Chapter 8 34

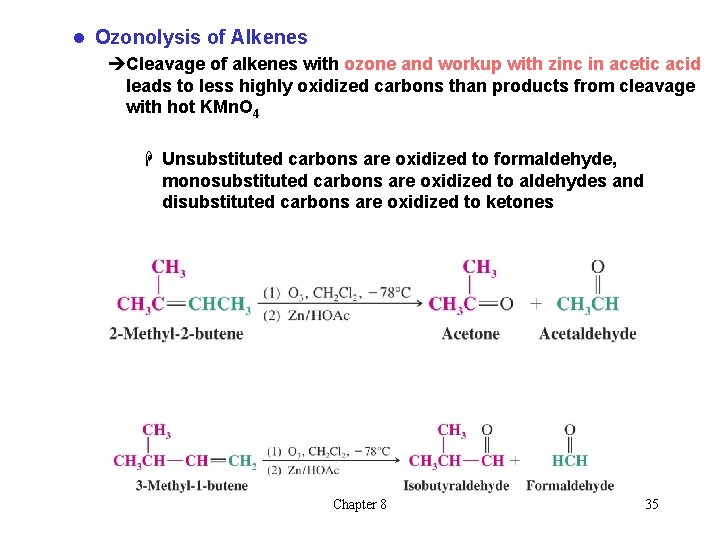
l Ozonolysis of Alkenes èCleavage of alkenes with ozone and workup with zinc in acetic acid leads to less highly oxidized carbons than products from cleavage with hot KMn. O 4 H Unsubstituted carbons are oxidized to formaldehyde, monosubstituted carbons are oxidized to aldehydes and disubstituted carbons are oxidized to ketones Chapter 8 35

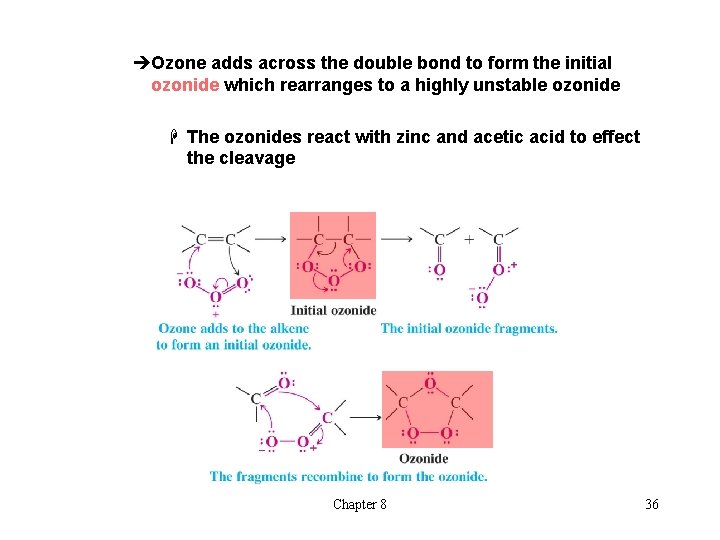
èOzone adds across the double bond to form the initial ozonide which rearranges to a highly unstable ozonide H The ozonides react with zinc and acetic acid to effect the cleavage Chapter 8 36

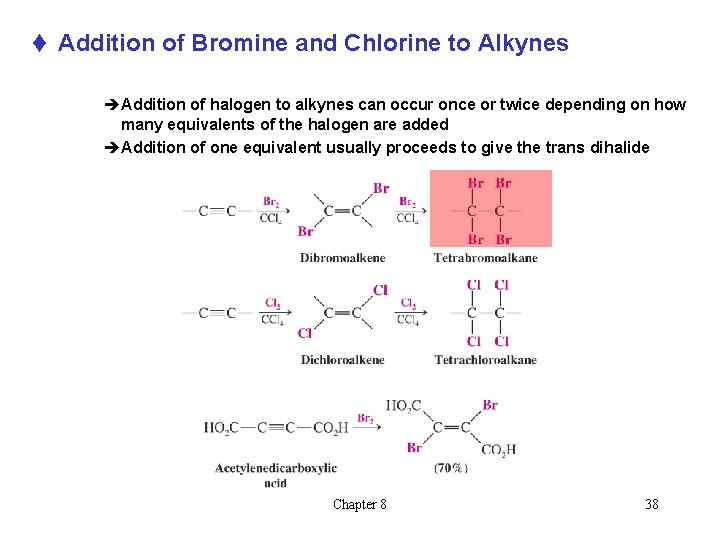
Addition of Bromine and Chlorine to Alkynes Chapter 8 37

t Addition of Bromine and Chlorine to Alkynes èAddition of halogen to alkynes can occur once or twice depending on how many equivalents of the halogen are added èAddition of one equivalent usually proceeds to give the trans dihalide Chapter 8 38

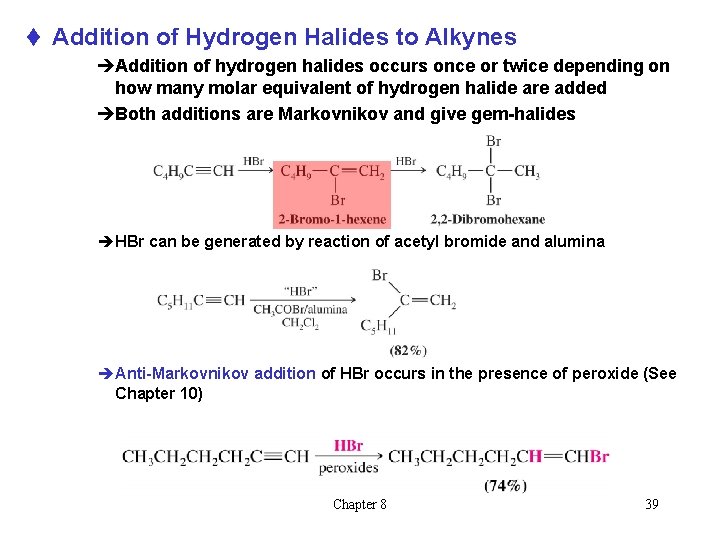
t Addition of Hydrogen Halides to Alkynes èAddition of hydrogen halides occurs once or twice depending on how many molar equivalent of hydrogen halide are added èBoth additions are Markovnikov and give gem-halides èHBr can be generated by reaction of acetyl bromide and alumina èAnti-Markovnikov addition of HBr occurs in the presence of peroxide (See Chapter 10) Chapter 8 39

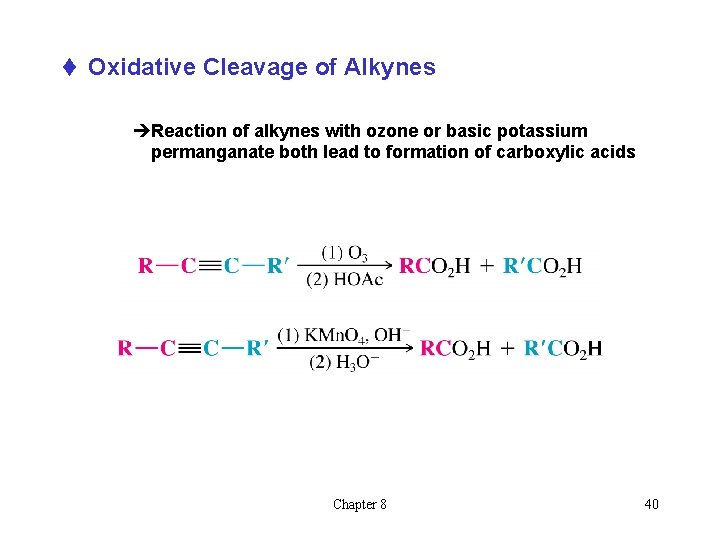
t Oxidative Cleavage of Alkynes èReaction of alkynes with ozone or basic potassium permanganate both lead to formation of carboxylic acids Chapter 8 40

Synthetic Strategies Revisited Chapter 8 41

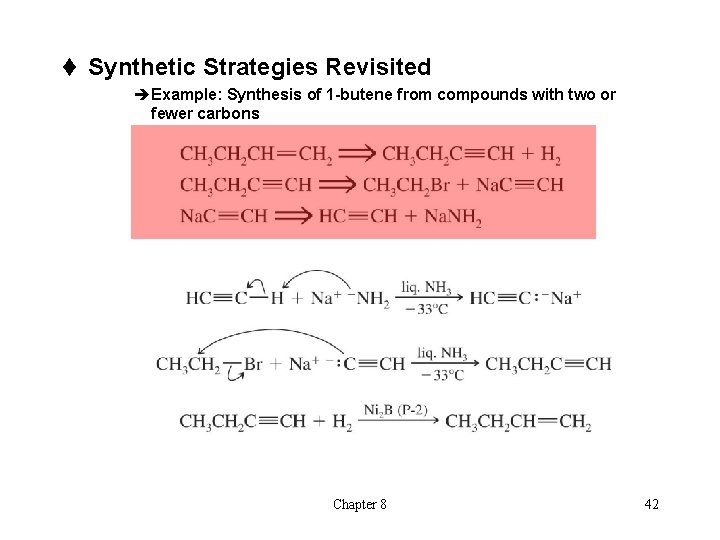
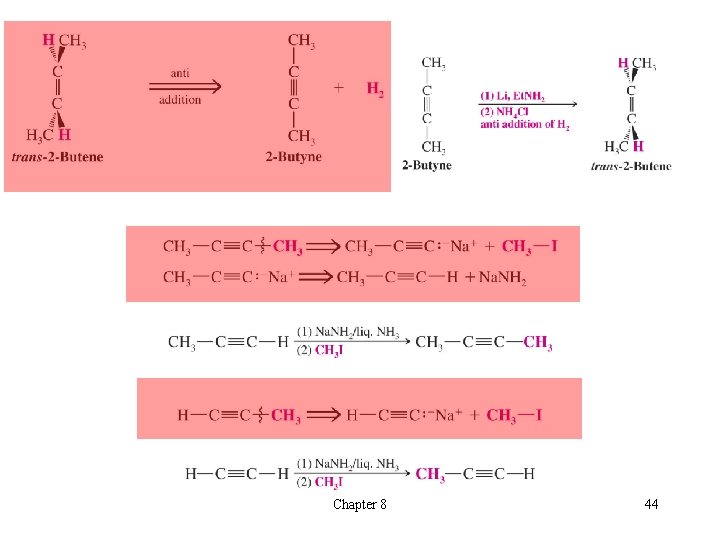
t Synthetic Strategies Revisited èExample: Synthesis of 1 -butene from compounds with two or fewer carbons Chapter 8 42

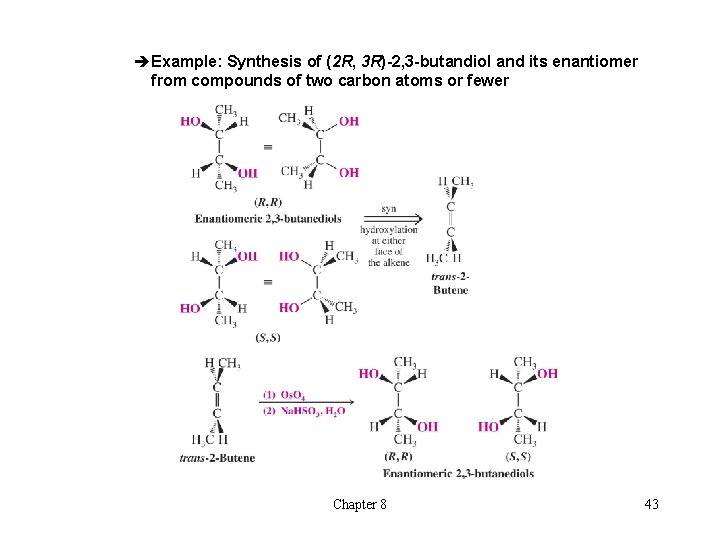
èExample: Synthesis of (2 R, 3 R)-2, 3 -butandiol and its enantiomer from compounds of two carbon atoms or fewer Chapter 8 43

Chapter 8 44
 Alkanes alkenes alkynes
Alkanes alkenes alkynes Chap chap slide
Chap chap slide Diol formation from alkene
Diol formation from alkene Halogenation of alkynes
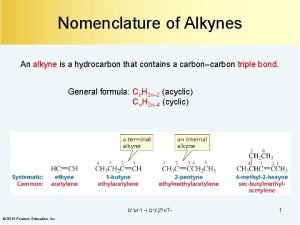
Halogenation of alkynes Triple bond nomenclature
Triple bond nomenclature Mechanism of ozonolysis
Mechanism of ozonolysis Addition of halogens to alkenes
Addition of halogens to alkenes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Alkynes
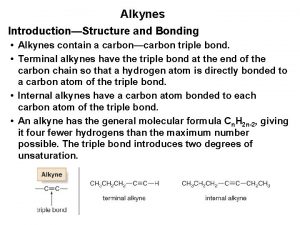
Alkynes Hybridization of alkynes
Hybridization of alkynes Condensed structural formula of propyne
Condensed structural formula of propyne Preparations of alkynes
Preparations of alkynes Alkynes
Alkynes Alkynes
Alkynes Hydroboration of alkynes
Hydroboration of alkynes First 10 members of alkynes
First 10 members of alkynes Claisen adapter
Claisen adapter Combustion of alkynes
Combustion of alkynes Properties of alkenes
Properties of alkenes Incomplete combustion of alkene
Incomplete combustion of alkene Alkene structural formula
Alkene structural formula Chemsheets reactions of halogenoalkanes 2
Chemsheets reactions of halogenoalkanes 2 Halogenation of alkenes
Halogenation of alkenes Physical properties of alkenes
Physical properties of alkenes Hybridization of alkenes
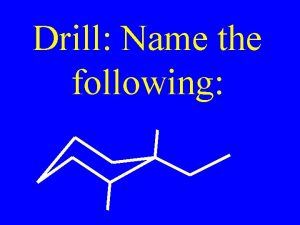
Hybridization of alkenes Name the following alkene:
Name the following alkene: Alkyne prefix
Alkyne prefix Alkanes in bromine water
Alkanes in bromine water Homologous series of hydrocarbons
Homologous series of hydrocarbons Ozonolysis
Ozonolysis Alkenes introduction
Alkenes introduction Riddles related to spices
Riddles related to spices 패션 chap 1
패션 chap 1 Passion chap 6
Passion chap 6 Bank run chap 11
Bank run chap 11 Assumptions of clrm gujarati
Assumptions of clrm gujarati Kstn chap 18
Kstn chap 18 Close family - chapter 3
Close family - chapter 3 The origin of species manwha
The origin of species manwha Satisfying needs chapter 1
Satisfying needs chapter 1 The origin of species 22 manga
The origin of species 22 manga Mad dog chap 25
Mad dog chap 25 If your right hand offends you
If your right hand offends you Chapter 1 learning about children
Chapter 1 learning about children