Chap 2 Alkanes Nomenclature Conformational Analysis and an








- Slides: 45

Chap. 2 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis (Textbook: Chapter 4)

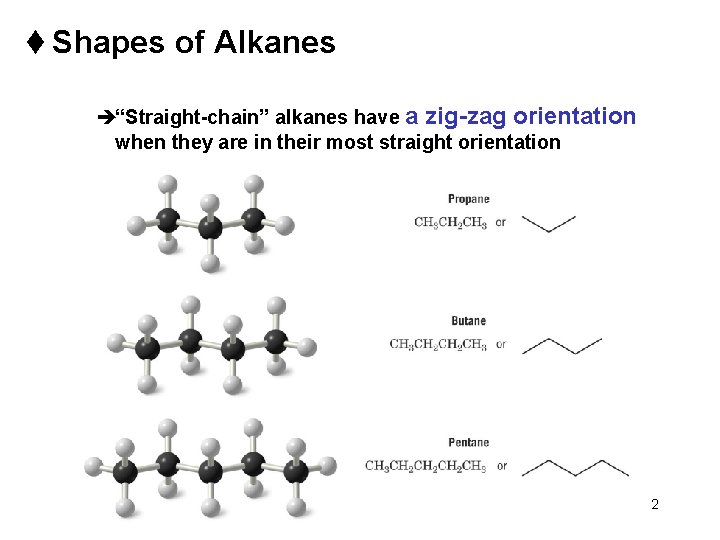
t Shapes of Alkanes è“Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation Chapter 4 2

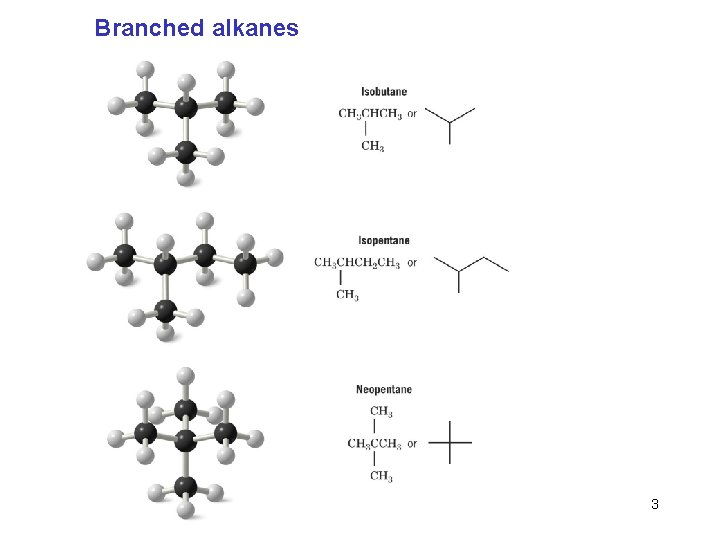
Branched alkanes Chapter 4 3

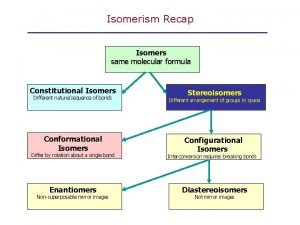
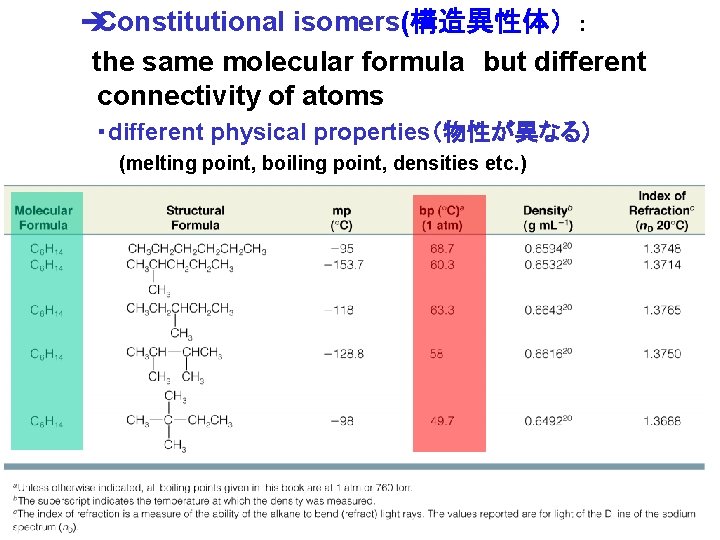
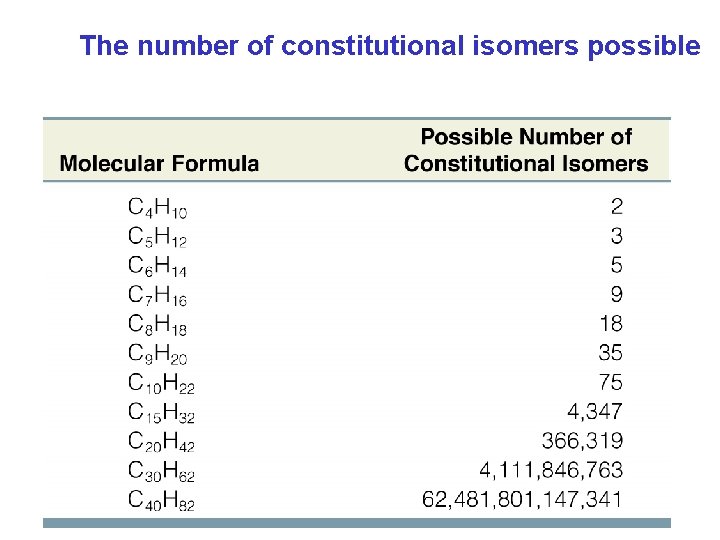
èConstitutional isomers(構造異性体) : the same molecular formula but different connectivity of atoms ・different physical properties(物性が異なる) (melting point, boiling point, densities etc. ) Chapter 4 4

The number of constitutional isomers possible Chapter 4 5

t. IUPAC Nomenclature of Alkanes, Alkyl Halides and Alcohols Chapter 4 6

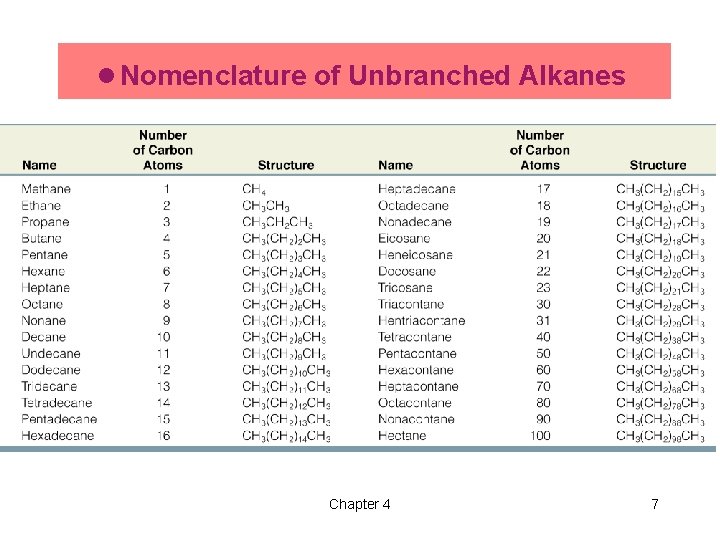
l Nomenclature of Unbranched Alkanes Chapter 4 7

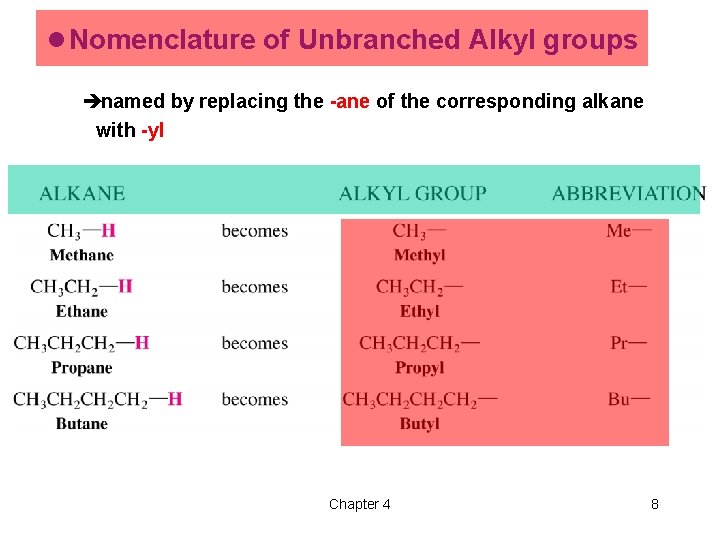
l Nomenclature of Unbranched Alkyl groups ènamed by replacing the -ane of the corresponding alkane with -yl Chapter 4 8

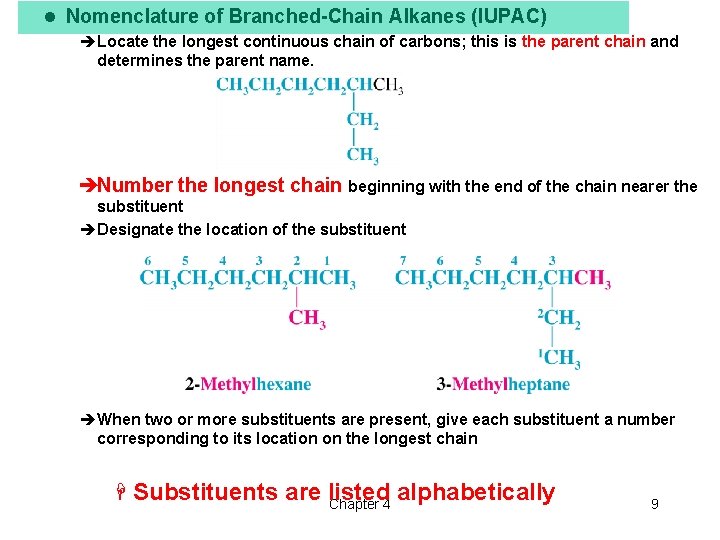
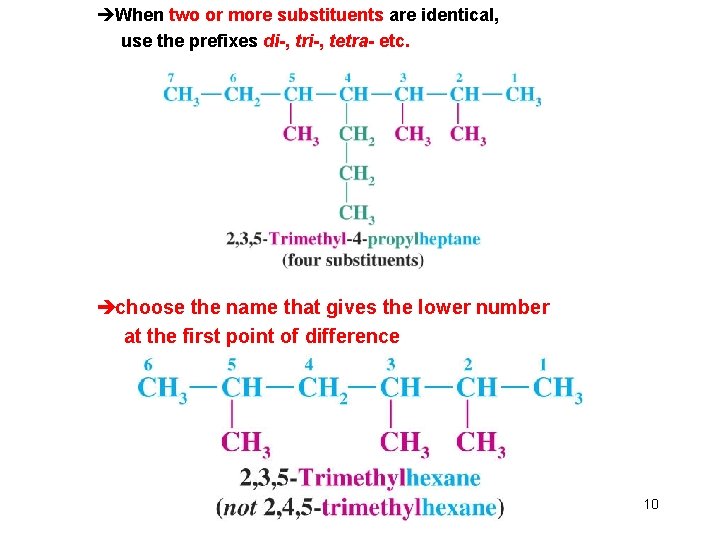
l Nomenclature of Branched-Chain Alkanes (IUPAC) èLocate the longest continuous chain of carbons; this is the parent chain and determines the parent name. èNumber the longest chain beginning with the end of the chain nearer the substituent èDesignate the location of the substituent èWhen two or more substituents are present, give each substituent a number corresponding to its location on the longest chain H Substituents are listed alphabetically Chapter 4 9

èWhen two or more substituents are identical, use the prefixes di-, tri-, tetra- etc. èchoose the name that gives the lower number at the first point of difference Chapter 4 10

l Nomenclature of Branched Alkyl Chains èTwo alkyl groups can be derived from propane Chapter 4 11

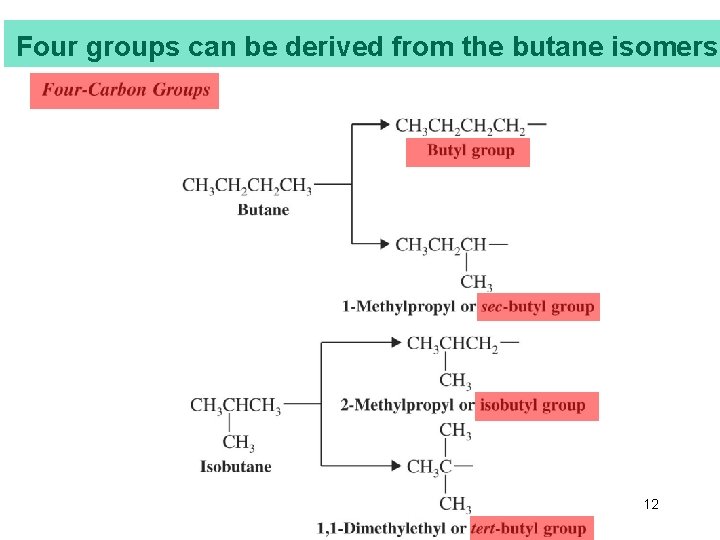
Four groups can be derived from the butane isomers Chapter 4 12

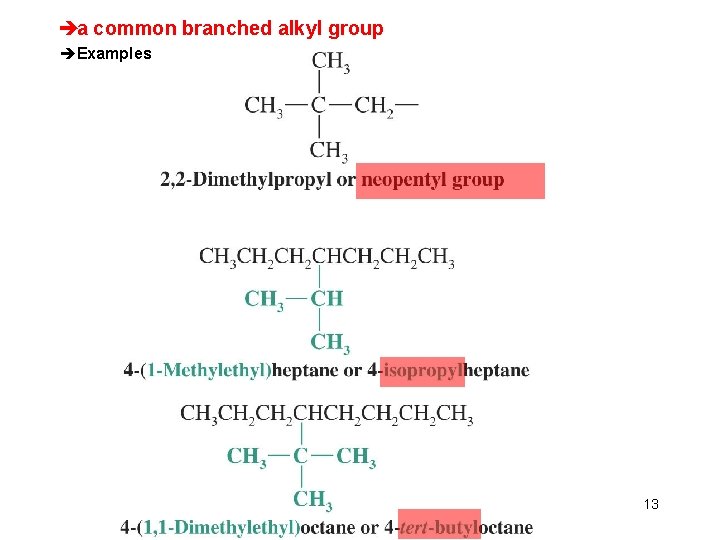
èa common branched alkyl group èExamples Chapter 4 13

t Classification of Hydrogen Atoms èHydrogens take their classification from the carbon they are attached to

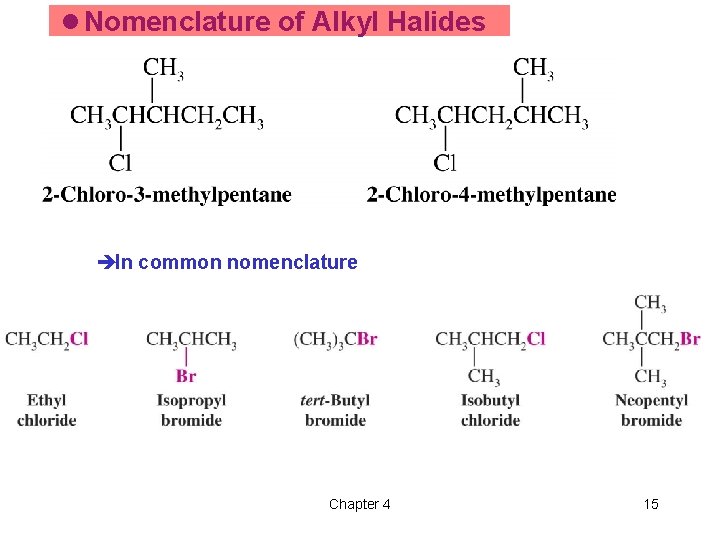
l Nomenclature of Alkyl Halides èIn common nomenclature Chapter 4 15

l IUPAC Substitutive Nomenclature èAn IUPAC name may have up to 4 features: locants, prefixes, parent compound and suffixes èNumbering generally starts from the end of the chain which is closest to the group named in the suffix èSelect the longest chain containing the hydroxyl and change the suffix name of the corresponding parent alkane from -ane to –ol èNumber the parent to give the hydroxyl the lowest possible number èThe other substituents take their locations accordingly Chapter 4 16

èIUPAC Nomenclature of Alcohols èCommon Names Chapter 4 17

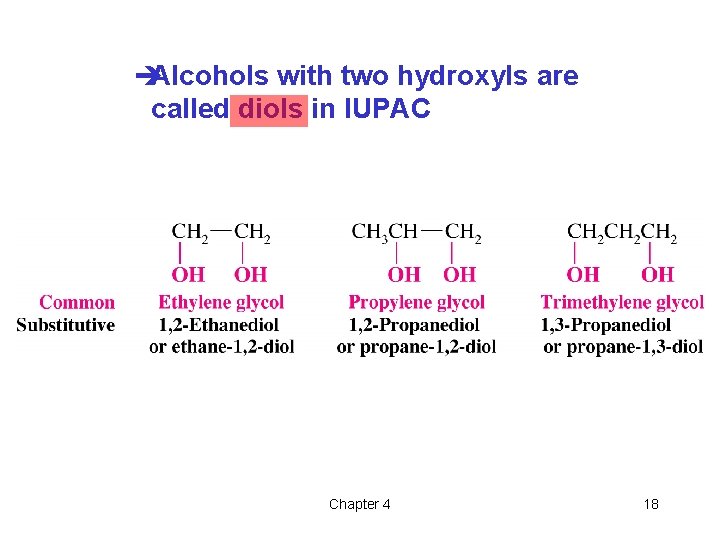
èAlcohols with two hydroxyls are called diols in IUPAC Chapter 4 18

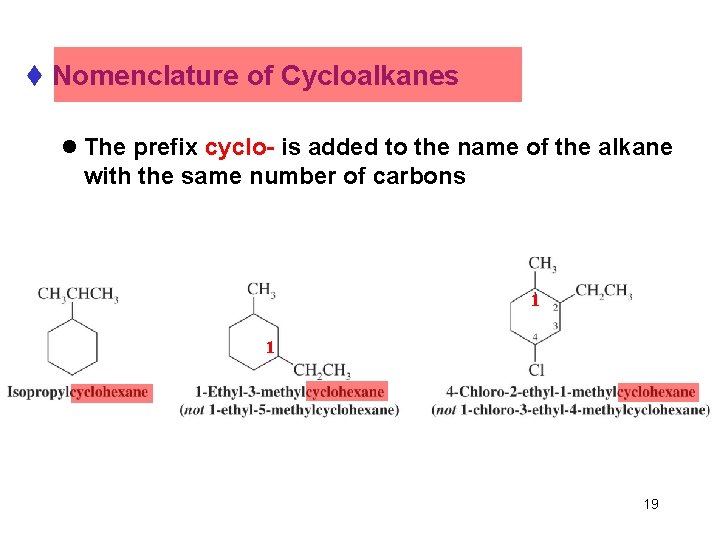
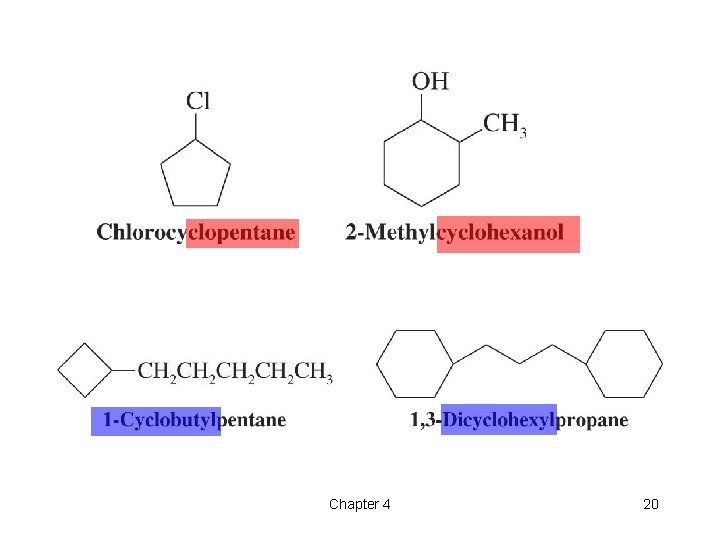
t Nomenclature of Cycloalkanes l The prefix cyclo- is added to the name of the alkane with the same number of carbons 1 1 19

Chapter 4 20

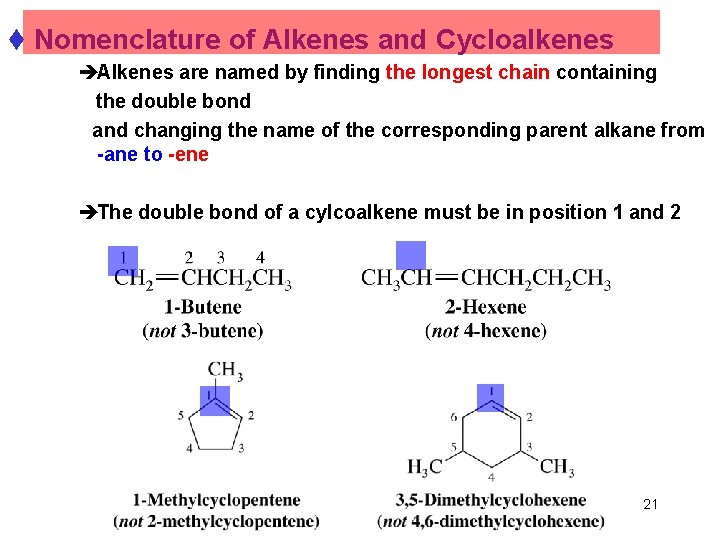
t Nomenclature of Alkenes and Cycloalkenes èAlkenes are named by finding the longest chain containing the double bond and changing the name of the corresponding parent alkane from -ane to -ene èThe double bond of a cylcoalkene must be in position 1 and 2 Chapter 4 21

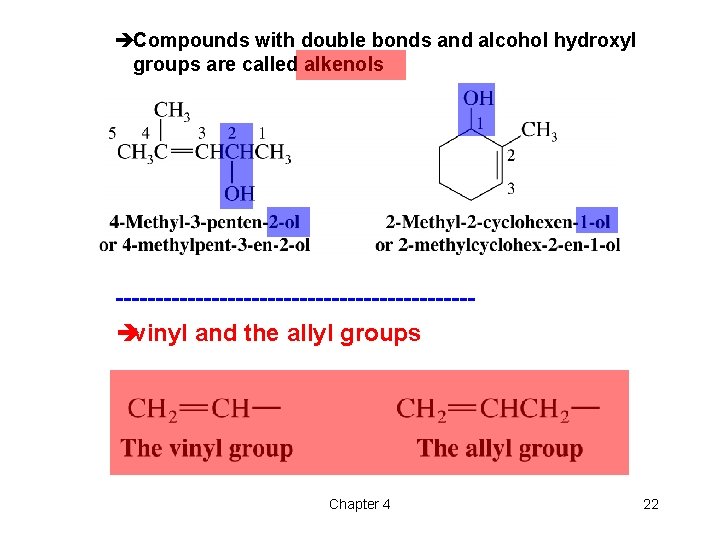
èCompounds with double bonds and alcohol hydroxyl groups are called alkenols ----------------------èvinyl and the allyl groups Chapter 4 22

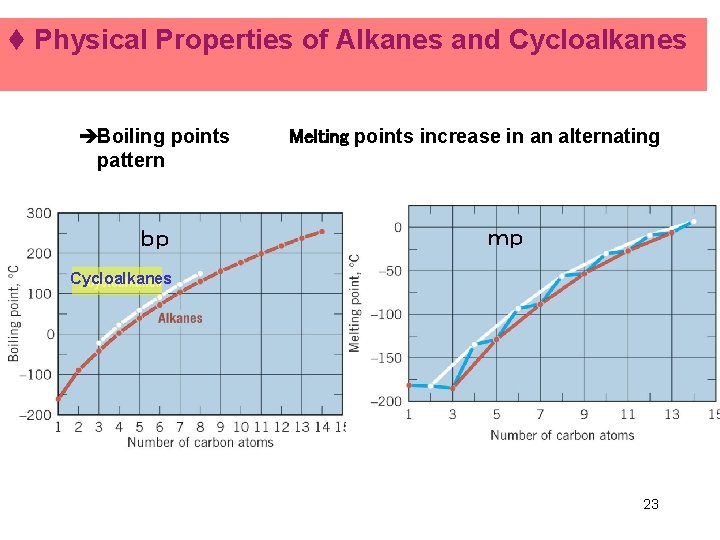
t Physical Properties of Alkanes and Cycloalkanes èBoiling points Melting points increase in an alternating pattern bp mp Cycloalkanes è 23

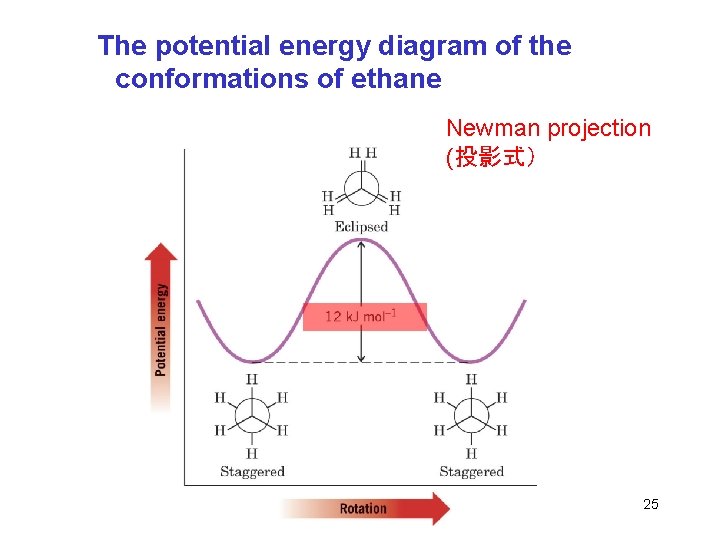
t Sigma Bonds and Bond Rotation èEthane has relatively free rotation around the carbon-carbon bond èThe staggered conformation has C-H bonds on adjacent carbons as far apart from each other as possible H Newman projection èThe eclipsed conformation has all C-H bonds on adjacent carbons directly on top of each other Chapter 4 24

The potential energy diagram of the conformations of ethane Newman projection (投影式) Chapter 4 25

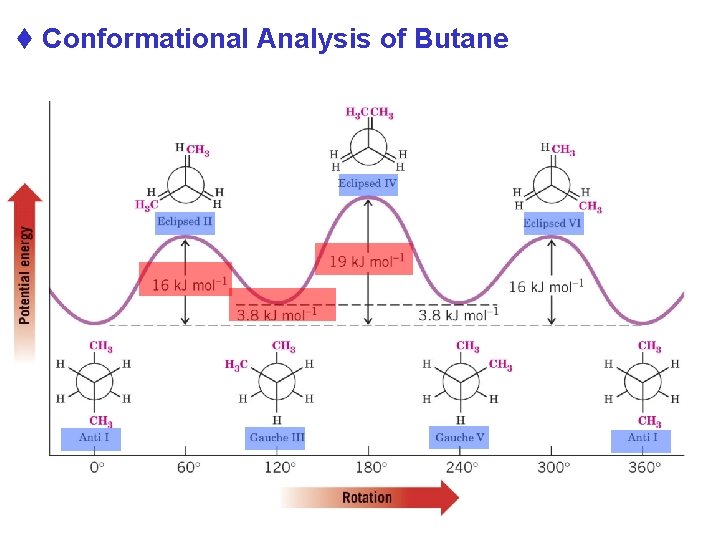
t Conformational Analysis of Butane Chapter 4 26

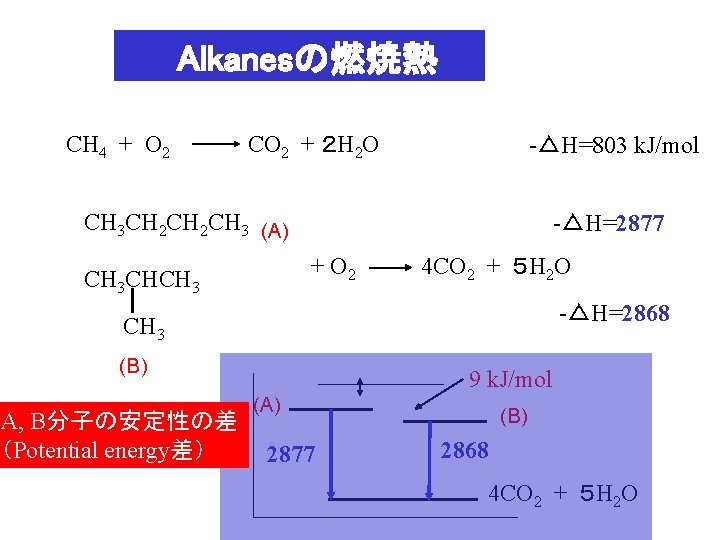
Alkanesの燃焼熱 CH 4 + O 2 CO 2 + 2 H 2 O -△H=803 k. J/mol CH 3 CH 2 CH 3 (A) -△H=2877 + O 2 CH 3 CHCH 3 4 CO 2 + 5 H 2 O -△H=2868 CH 3 (B) A, B分子の安定性の差 (Potential energy差) 9 k. J/mol (A) 2877 (B) 2868 4 CO 2 + 5 H 2 O

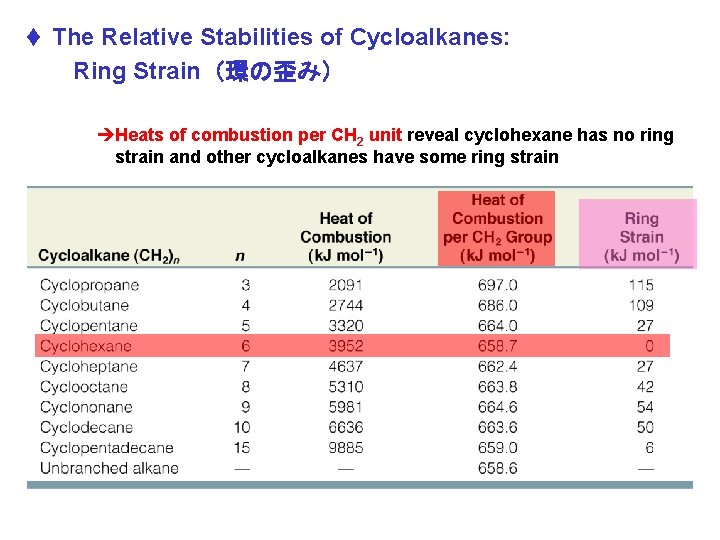
t The Relative Stabilities of Cycloalkanes: Ring Strain(環の歪み) èHeats of combustion per CH 2 unit reveal cyclohexane has no ring strain and other cycloalkanes have some ring strain

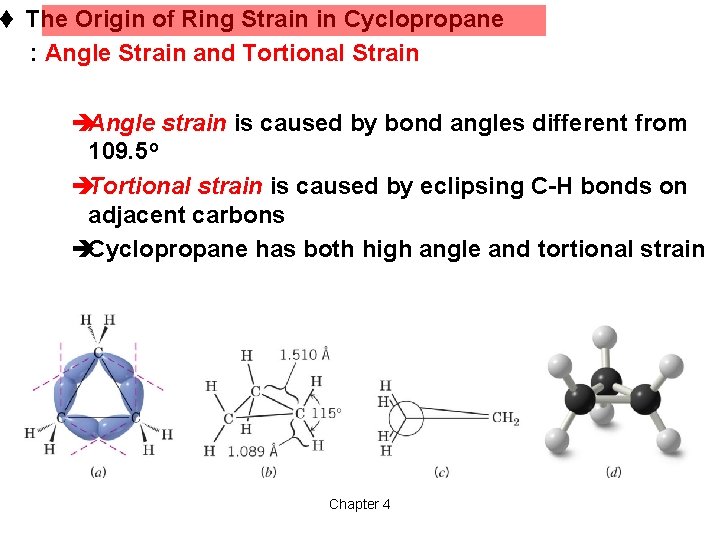
t The Origin of Ring Strain in Cyclopropane : Angle Strain and Tortional Strain èAngle strain is caused by bond angles different from 109. 5 o èTortional strain is caused by eclipsing C-H bonds on adjacent carbons èCyclopropane has both high angle and tortional strain Chapter 4

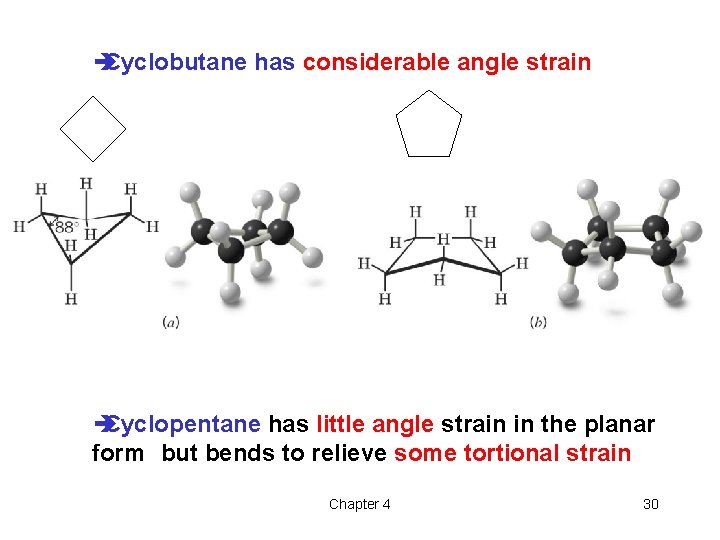
è Cyclobutane has considerable angle strain è Cyclopentane has little angle strain in the planar form but bends to relieve some tortional strain Chapter 4 30

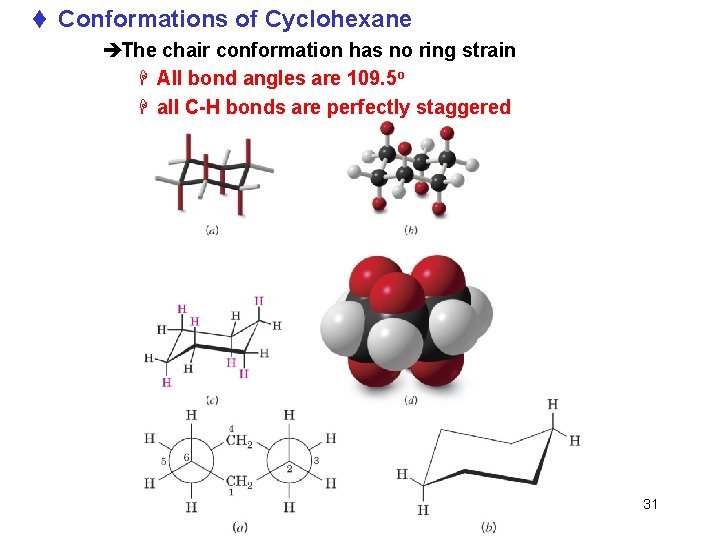
t Conformations of Cyclohexane èThe chair conformation has no ring strain H All bond angles are 109. 5 o H all C-H bonds are perfectly staggered Chapter 4 31

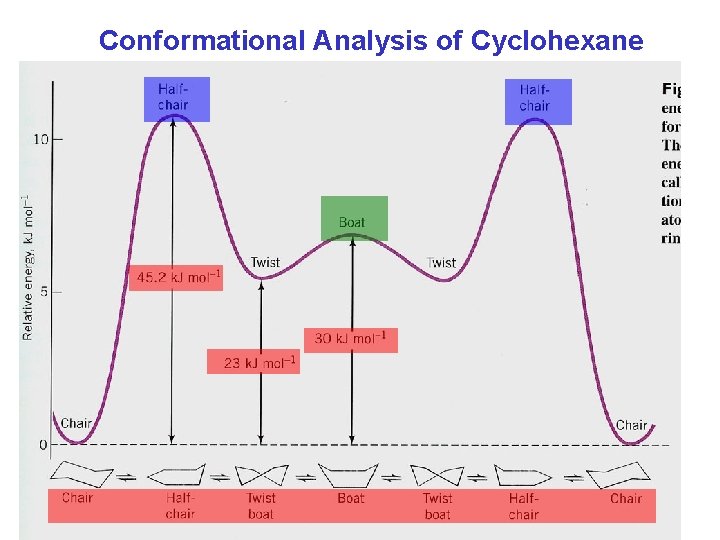
Conformational Analysis of Cyclohexane Chapter 4 32

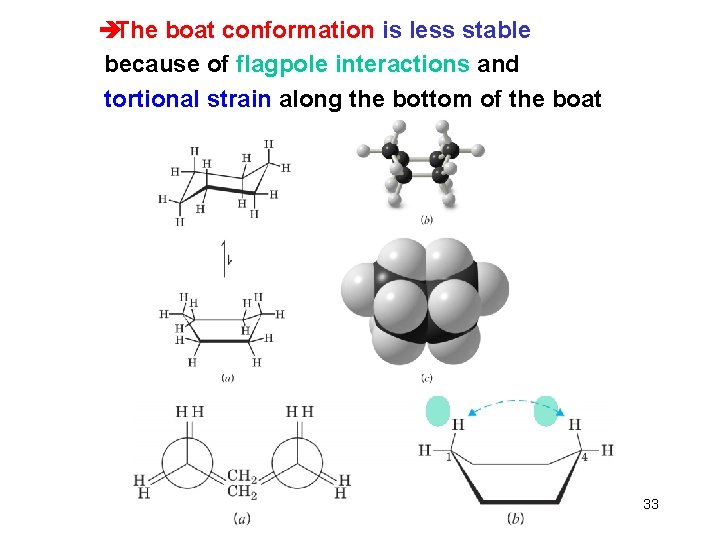
èThe boat conformation is less stable because of flagpole interactions and tortional strain along the bottom of the boat Chapter 4 33

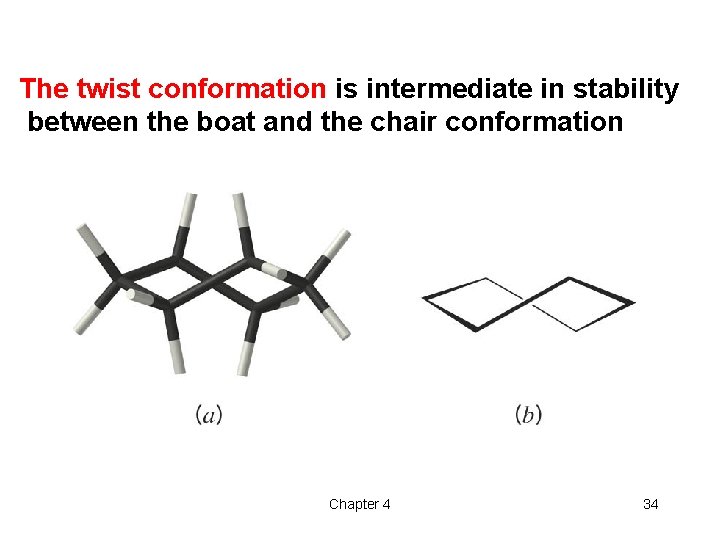
The twist conformation is intermediate in stability between the boat and the chair conformation Chapter 4 34

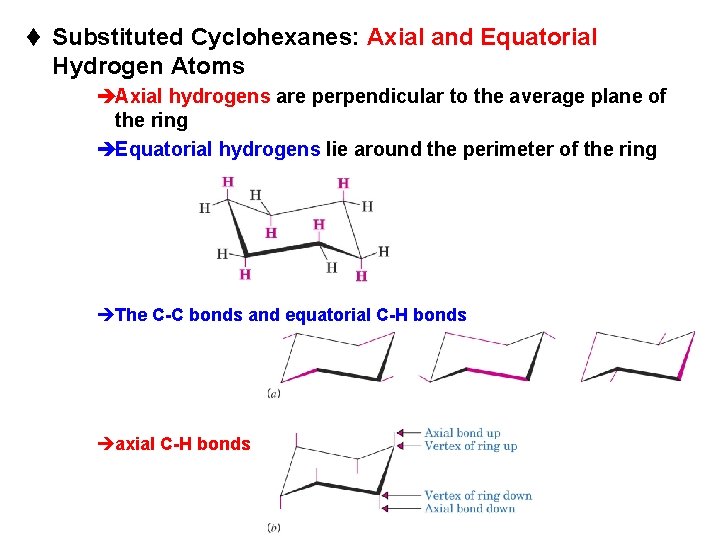
t Substituted Cyclohexanes: Axial and Equatorial Hydrogen Atoms èAxial hydrogens are perpendicular to the average plane of the ring èEquatorial hydrogens lie around the perimeter of the ring èThe C-C bonds and equatorial C-H bonds èaxial C-H bonds Chapter 4 35

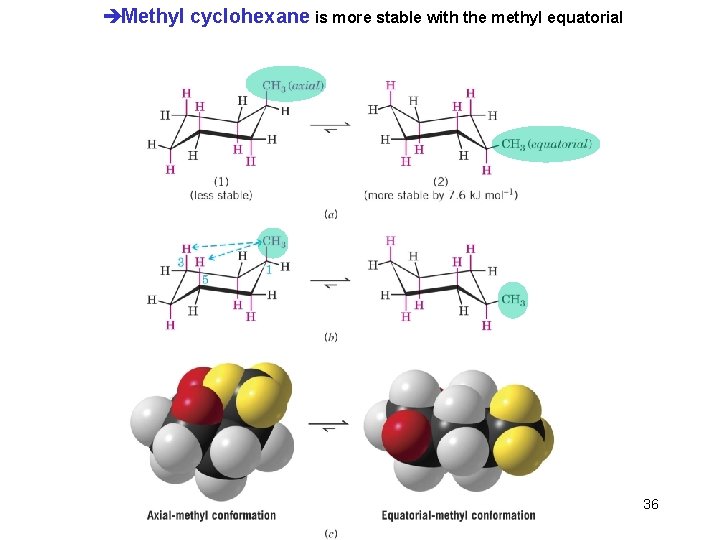
èMethyl cyclohexane is more stable with the methyl equatorial Chapter 4 36

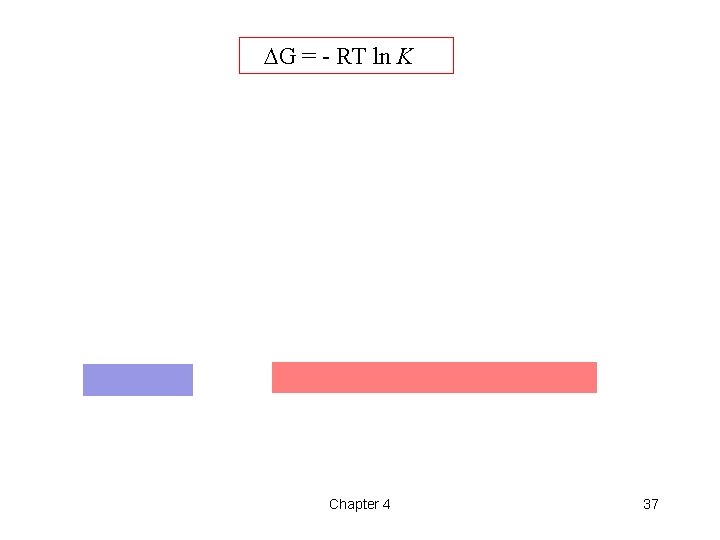
DG = - RT ln K Chapter 4 37

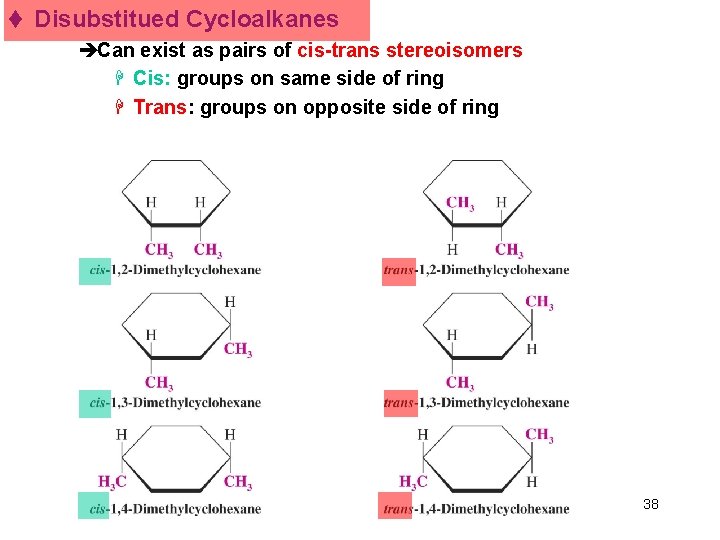
t Disubstitued Cycloalkanes èCan exist as pairs of cis-trans stereoisomers H Cis: groups on same side of ring H Trans: groups on opposite side of ring Chapter 4 38

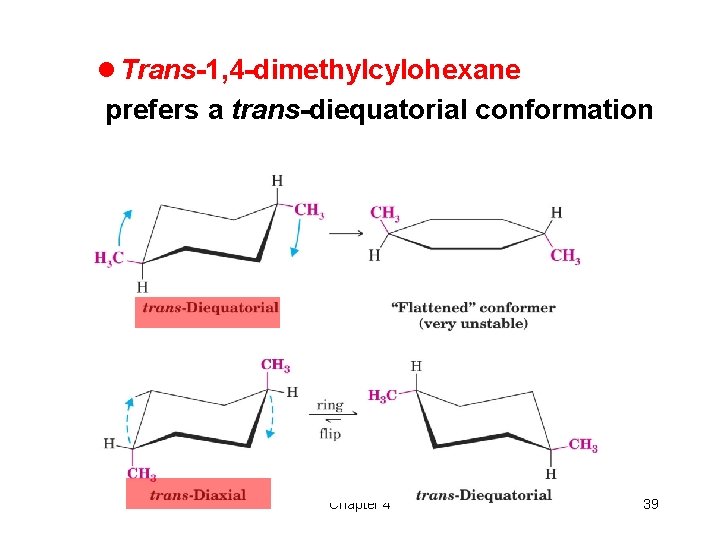
l Trans-1, 4 -dimethylcylohexane prefers a trans-diequatorial conformation Chapter 4 39

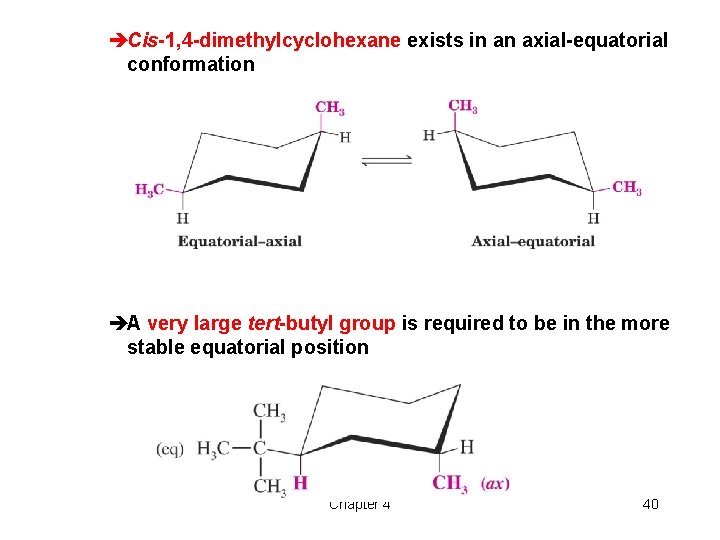
èCis-1, 4 -dimethylcyclohexane exists in an axial-equatorial conformation èA very large tert-butyl group is required to be in the more stable equatorial position Chapter 4 40

t Bicyclic and Polycyclic Alkanes èThe bicyclic decalin system exists in non-interconvertible cis and trans forms Chapter 4 41

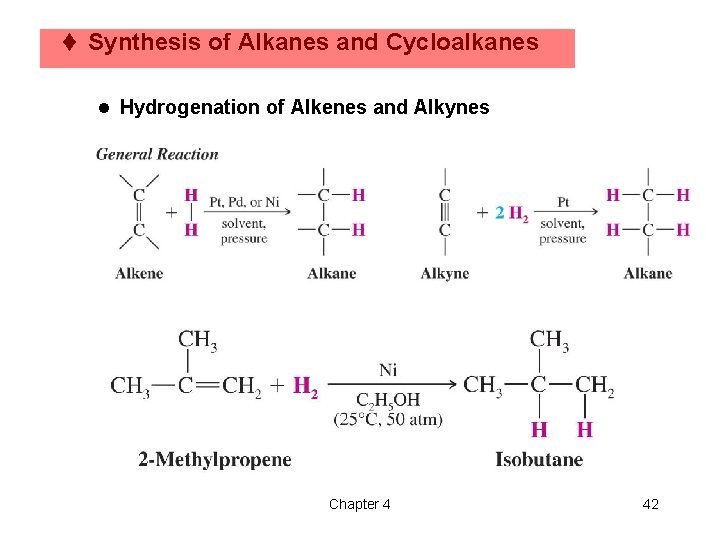
t Synthesis of Alkanes and Cycloalkanes l Hydrogenation of Alkenes and Alkynes Chapter 4 42

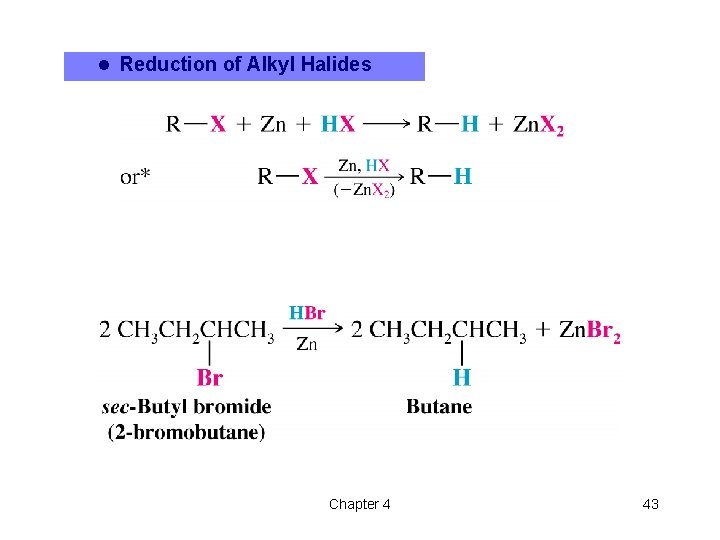
l Reduction of Alkyl Halides Chapter 4 43

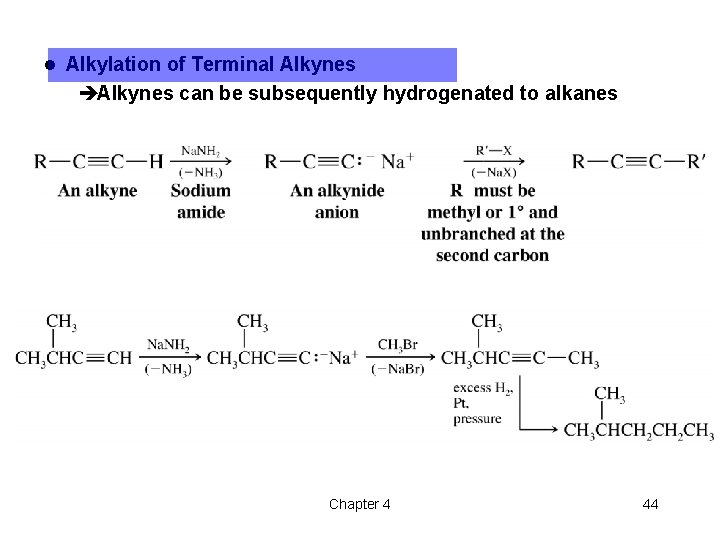
l Alkylation of Terminal Alkynes èAlkynes can be subsequently hydrogenated to alkanes Chapter 4 44

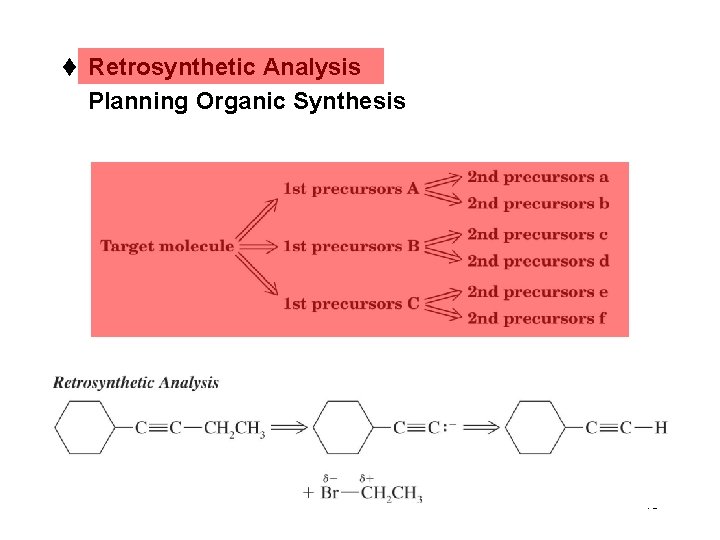
t Retrosynthetic Analysis Planning Organic Synthesis 45
 Ch3ch2cooch2ch3
Ch3ch2cooch2ch3 Chap chap slide
Chap chap slide Constitutional isomer vs stereoisomer
Constitutional isomer vs stereoisomer Conformational isomers
Conformational isomers Non superimposable mirror images are
Non superimposable mirror images are Bond angle in cyclohexane
Bond angle in cyclohexane I was in that state when a chap easily turns nasty analysis
I was in that state when a chap easily turns nasty analysis Alkanes solubility
Alkanes solubility Isomers of pentane
Isomers of pentane Explain homologous series with example
Explain homologous series with example Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list Uses of alkanes
Uses of alkanes Alkanes alkenes alkynes
Alkanes alkenes alkynes 3-butyl-3-propyl-1-pentyne
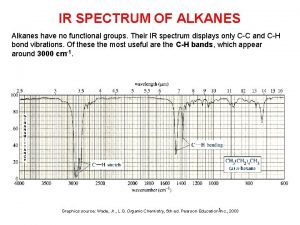
3-butyl-3-propyl-1-pentyne Alkanes ir spectrum
Alkanes ir spectrum Combustion of alkynes
Combustion of alkynes Alkane combustion
Alkane combustion Alkanes def
Alkanes def Alkylation of alkanes
Alkylation of alkanes Viscosity marble experiment
Viscosity marble experiment Relative stability of isomeric alkanes
Relative stability of isomeric alkanes Iupac stands for
Iupac stands for Bromine water test
Bromine water test Meth eth prop
Meth eth prop Uses of alkanes
Uses of alkanes Riddles of spices with answer
Riddles of spices with answer 1 of 1 clothing meaning
1 of 1 clothing meaning Passion chap 6
Passion chap 6 Bank run chap 11
Bank run chap 11 Autocorrelation in econometrics
Autocorrelation in econometrics Kstn chap 18
Kstn chap 18 Family values chapter 3
Family values chapter 3 The origin of species - chapter 24 manga buddy
The origin of species - chapter 24 manga buddy Satisfy needs and wants
Satisfy needs and wants The origin of species chapter 22 manhwa
The origin of species chapter 22 manhwa Mad dog chap 25
Mad dog chap 25 Matthew chapter 5
Matthew chapter 5 Chapter 1 learning about children
Chapter 1 learning about children Rivalry 1 chapter 6
Rivalry 1 chapter 6 System engineer chap 1
System engineer chap 1 Chap tree
Chap tree Kstn chap 7
Kstn chap 7 Gradualism vs punctuated equilibrium
Gradualism vs punctuated equilibrium Passion chapter 9
Passion chapter 9 Bài tập về nhà
Bài tập về nhà