Chap 18 Part 2 Centromere point of constriction





- Slides: 60

Chap. 18 Part 2

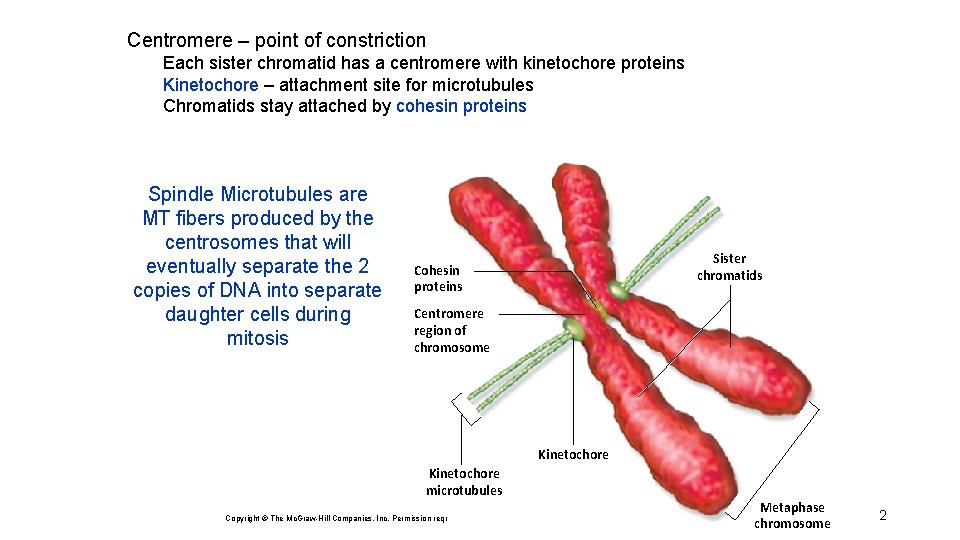
Centromere – point of constriction Each sister chromatid has a centromere with kinetochore proteins Kinetochore – attachment site for microtubules Chromatids stay attached by cohesin proteins Spindle Microtubules are MT fibers produced by the centrosomes that will eventually separate the 2 copies of DNA into separate daughter cells during mitosis Sister chromatids Cohesin proteins Centromere region of chromosome Kinetochore microtubules Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Metaphase chromosome 2

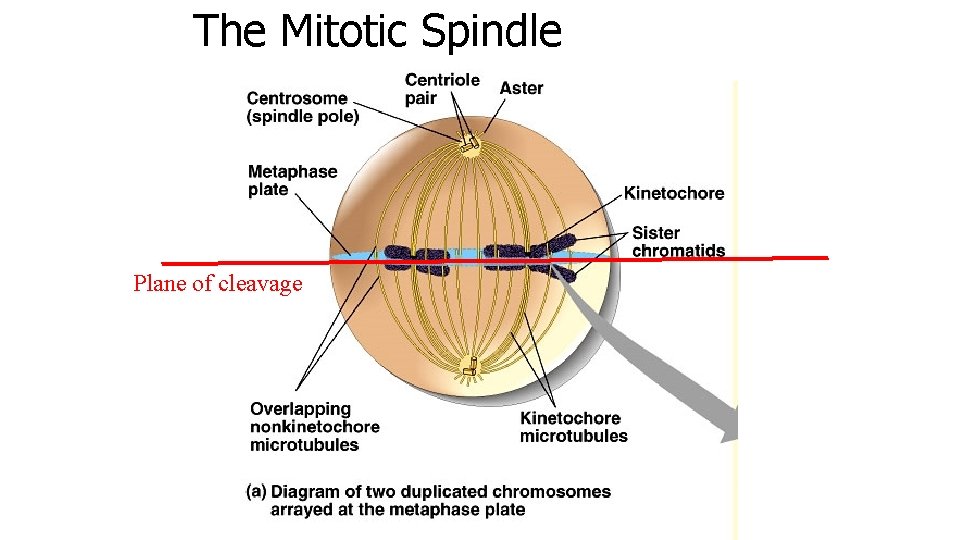
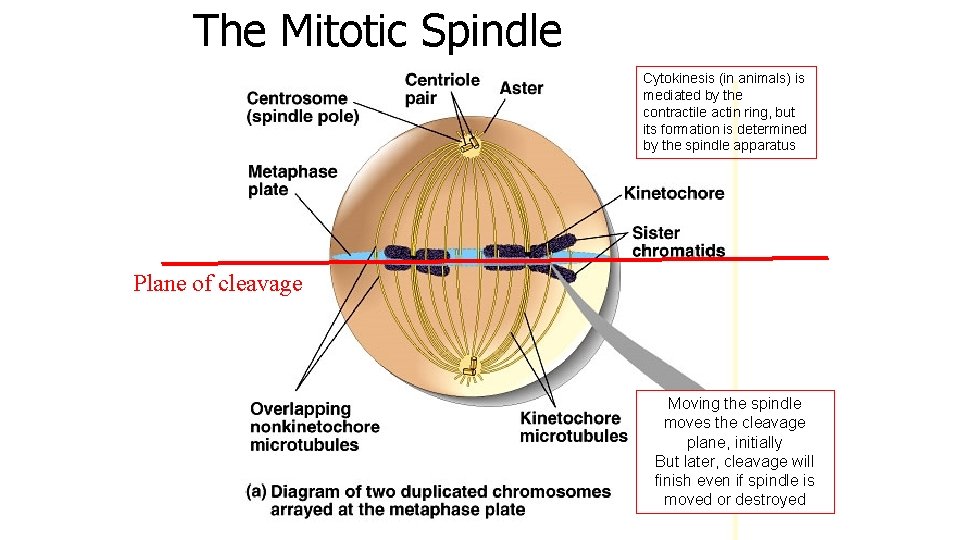
The Mitotic Spindle Plane of cleavage

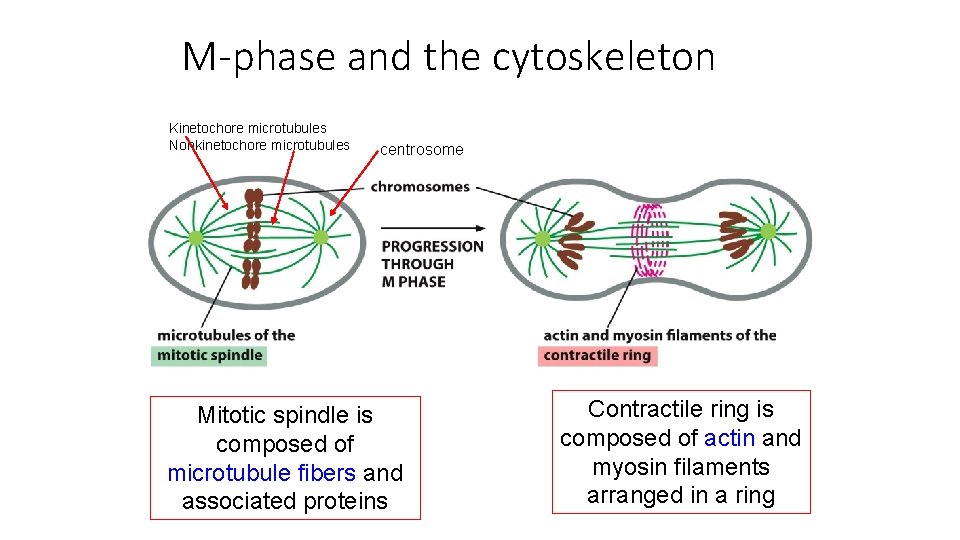
M-phase and the cytoskeleton Kinetochore microtubules Nonkinetochore microtubules centrosome Mitotic spindle is composed of microtubule fibers and associated proteins Contractile ring is composed of actin and myosin filaments arranged in a ring

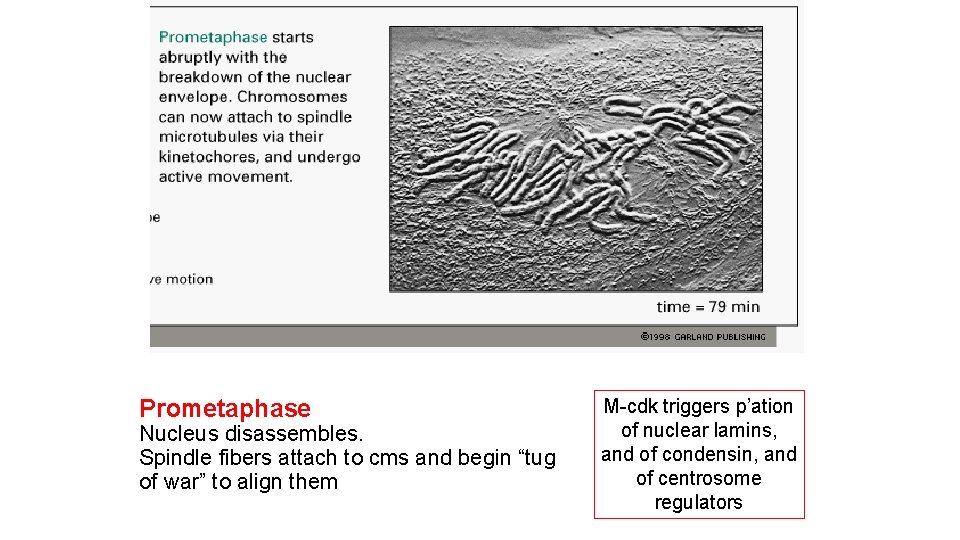
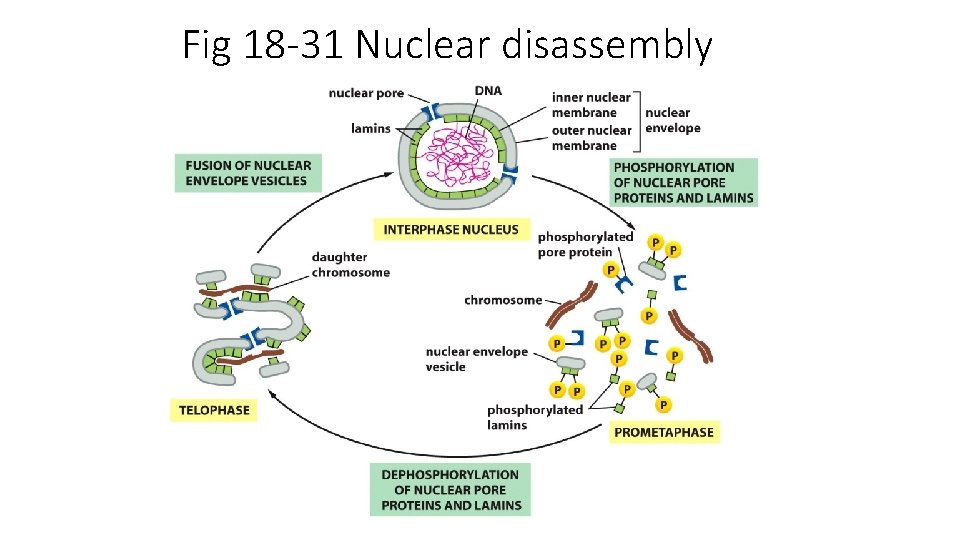
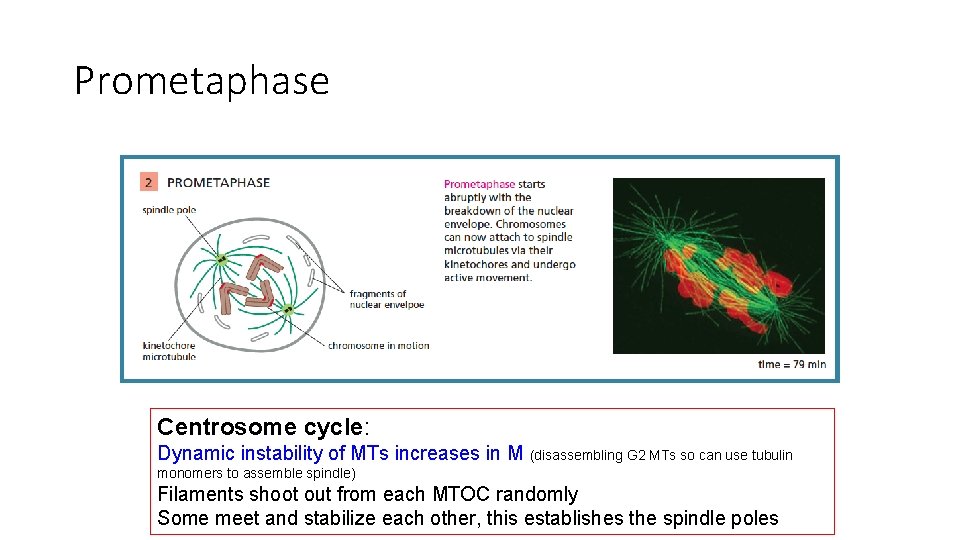
Prometaphase Nucleus disassembles. Spindle fibers attach to cms and begin “tug of war” to align them M-cdk triggers p’ation of nuclear lamins, and of condensin, and of centrosome regulators

Fig 18 -31 Nuclear disassembly

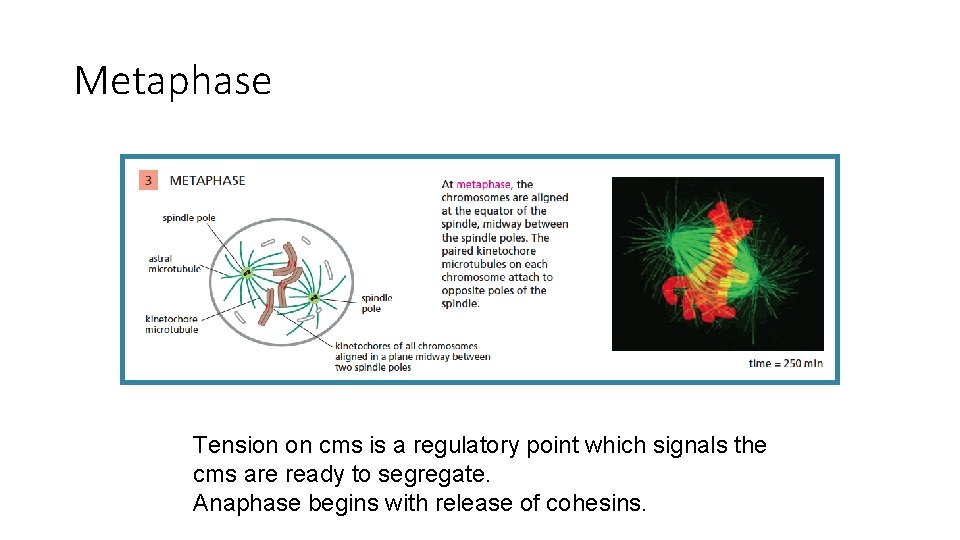
Metaphase chromosomes align at metaphase plate the spindle apparatus does this by applying tension on both sides of cms via microtubule spindles

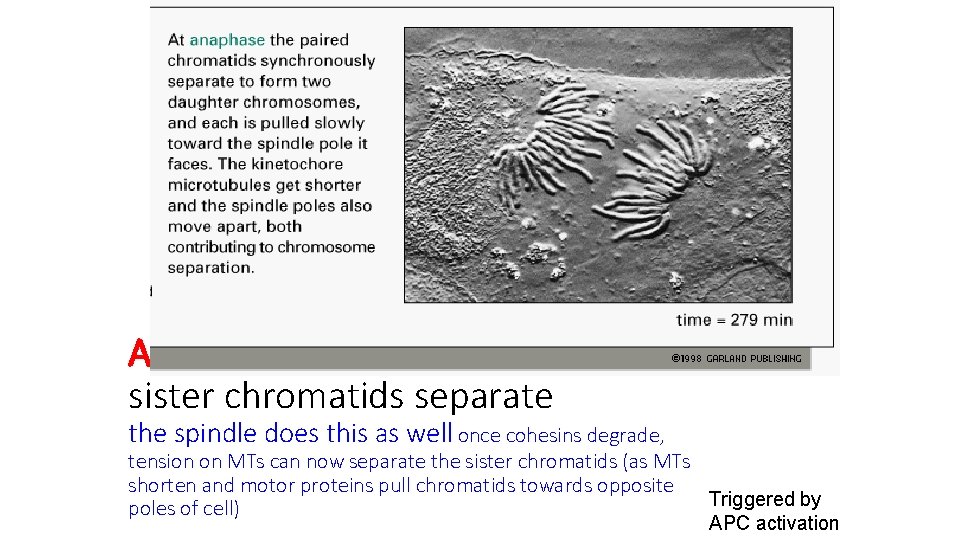
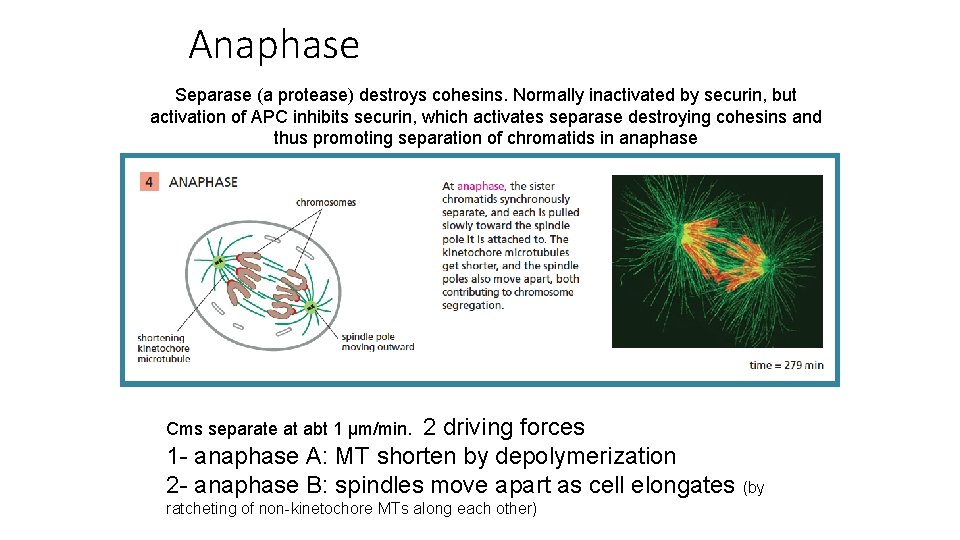
Anaphase sister chromatids separate the spindle does this as well once cohesins degrade, tension on MTs can now separate the sister chromatids (as MTs shorten and motor proteins pull chromatids towards opposite Triggered by poles of cell) APC activation

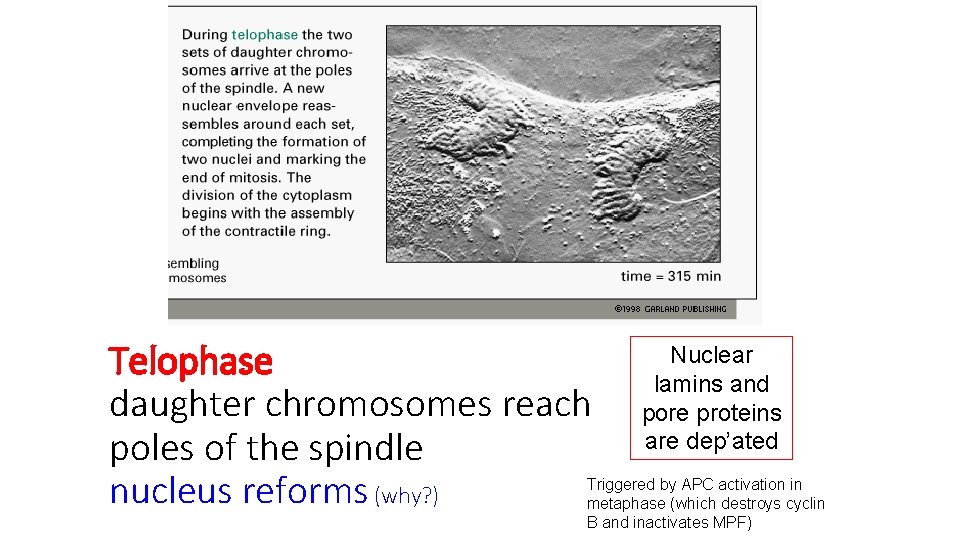
Telophase daughter chromosomes reach poles of the spindle nucleus reforms (why? ) Nuclear lamins and pore proteins are dep’ated Triggered by APC activation in metaphase (which destroys cyclin B and inactivates MPF)

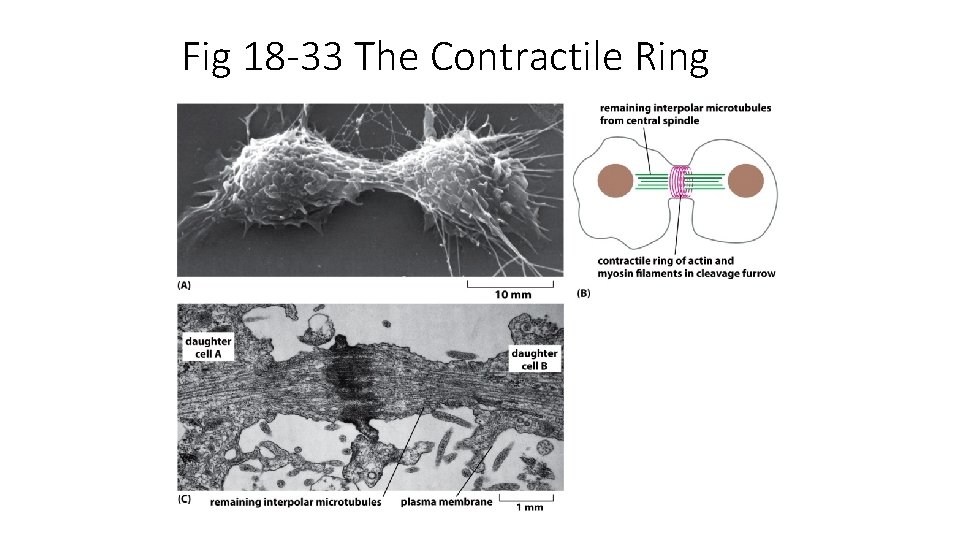
Cytokinesis (usually occurs at end of telophase) cytoplasm divides Due to contractile ring of actin and myosin fibers (forms along metaphase plate)

Cell division in plant cells Movie 18 -3

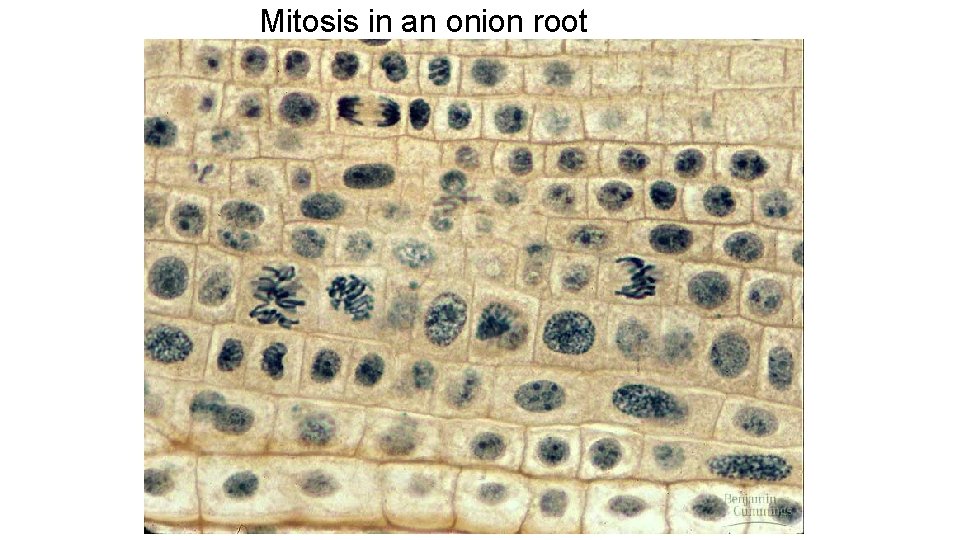
Mitosis in an onion root

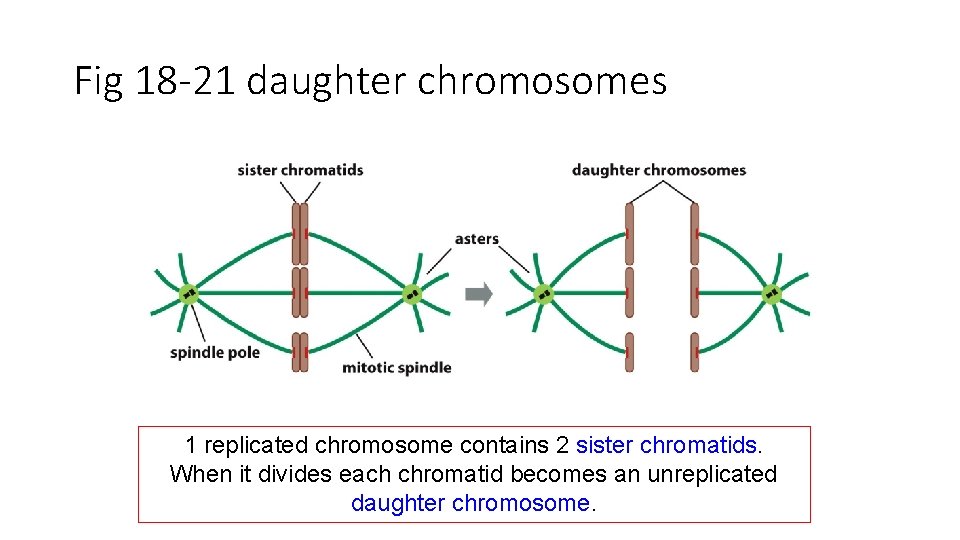
Fig 18 -21 daughter chromosomes 1 replicated chromosome contains 2 sister chromatids. When it divides each chromatid becomes an unreplicated daughter chromosome.

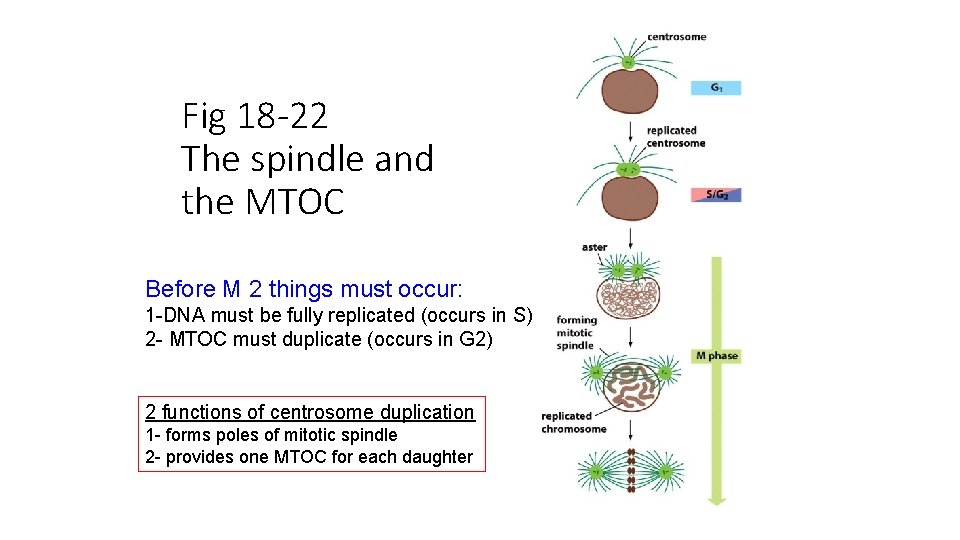
Fig 18 -22 The spindle and the MTOC Before M 2 things must occur: 1 -DNA must be fully replicated (occurs in S) 2 - MTOC must duplicate (occurs in G 2) 2 functions of centrosome duplication 1 - forms poles of mitotic spindle 2 - provides one MTOC for each daughter

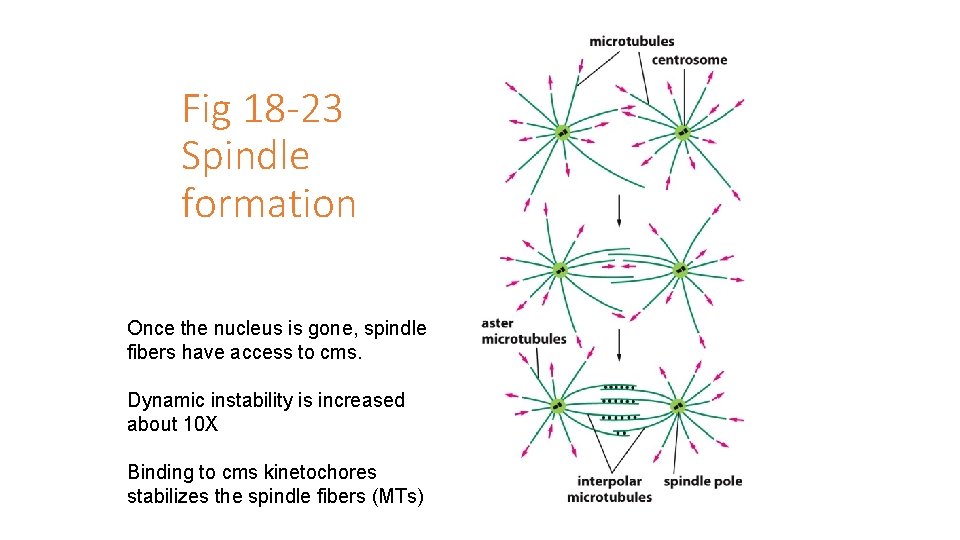
Fig 18 -23 Spindle formation Once the nucleus is gone, spindle fibers have access to cms. Dynamic instability is increased about 10 X Binding to cms kinetochores stabilizes the spindle fibers (MTs)

Prometaphase Centrosome cycle: Dynamic instability of MTs increases in M (disassembling G 2 MTs so can use tubulin monomers to assemble spindle) Filaments shoot out from each MTOC randomly Some meet and stabilize each other, this establishes the spindle poles

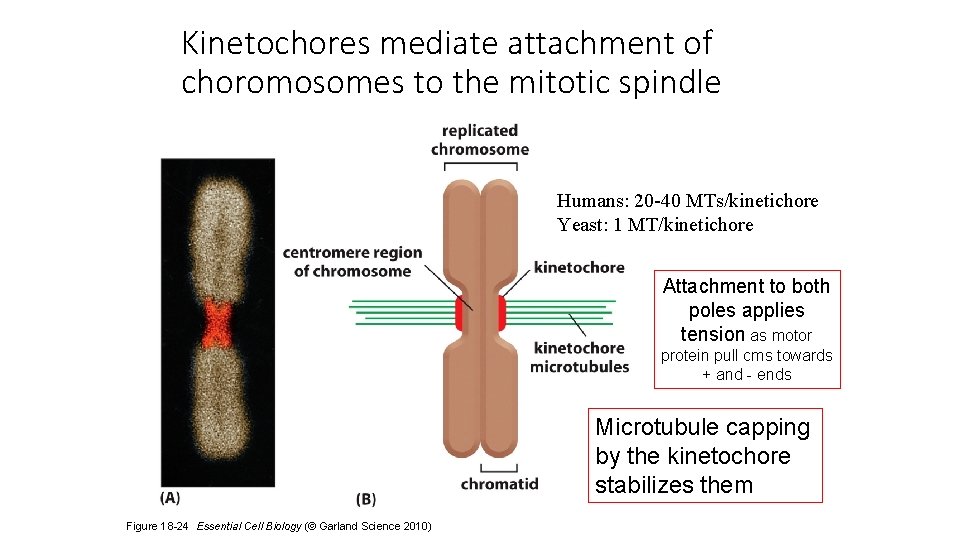
Kinetochores mediate attachment of choromosomes to the mitotic spindle Humans: 20 -40 MTs/kinetichore Yeast: 1 MT/kinetichore Attachment to both poles applies tension as motor protein pull cms towards + and - ends Microtubule capping by the kinetochore stabilizes them Figure 18 -24 Essential Cell Biology (© Garland Science 2010)

Metaphase Tension on cms is a regulatory point which signals the cms are ready to segregate. Anaphase begins with release of cohesins.

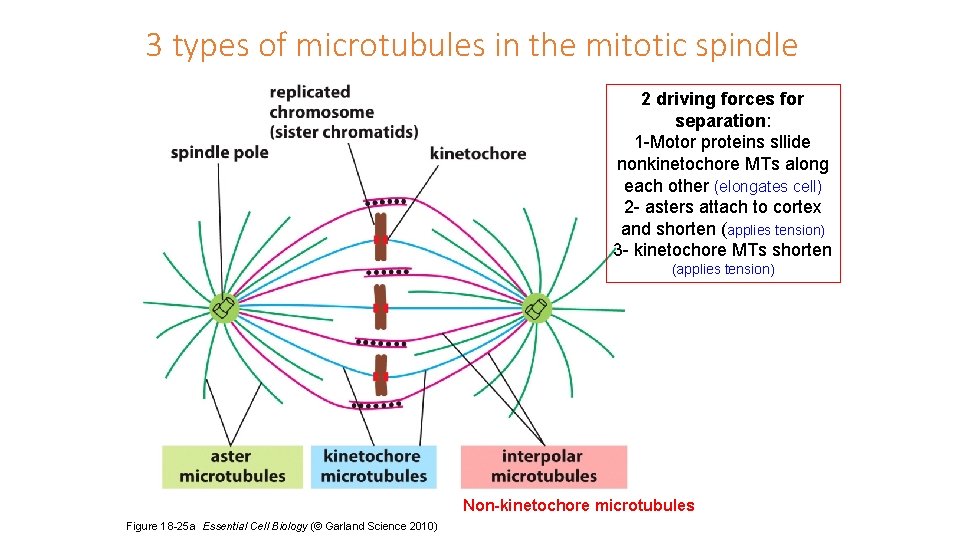
3 types of microtubules in the mitotic spindle 2 driving forces for separation: 1 -Motor proteins sllide nonkinetochore MTs along each other (elongates cell) 2 - asters attach to cortex and shorten (applies tension) 3 - kinetochore MTs shorten (applies tension) Non-kinetochore microtubules Figure 18 -25 a Essential Cell Biology (© Garland Science 2010)

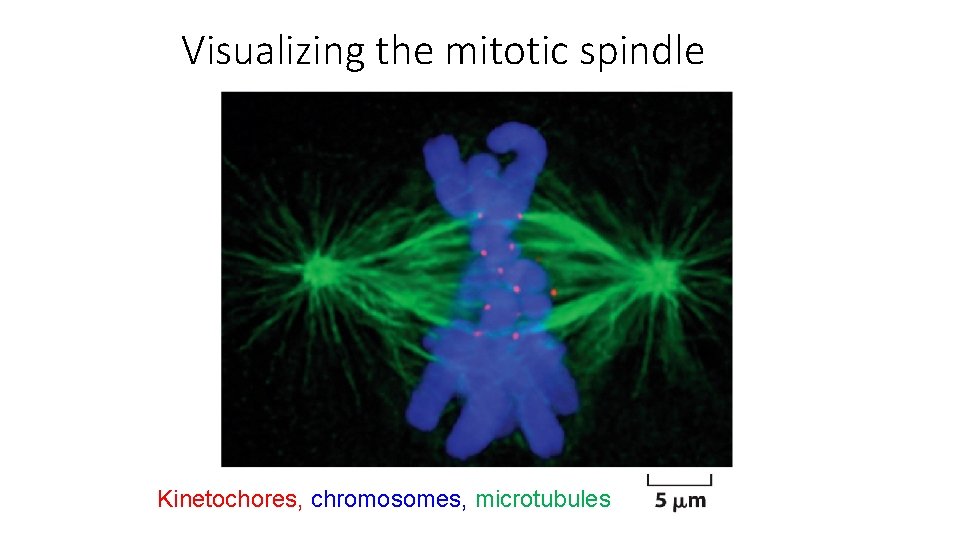
Visualizing the mitotic spindle Kinetochores, chromosomes, microtubules

Spindle Movie 18 -5

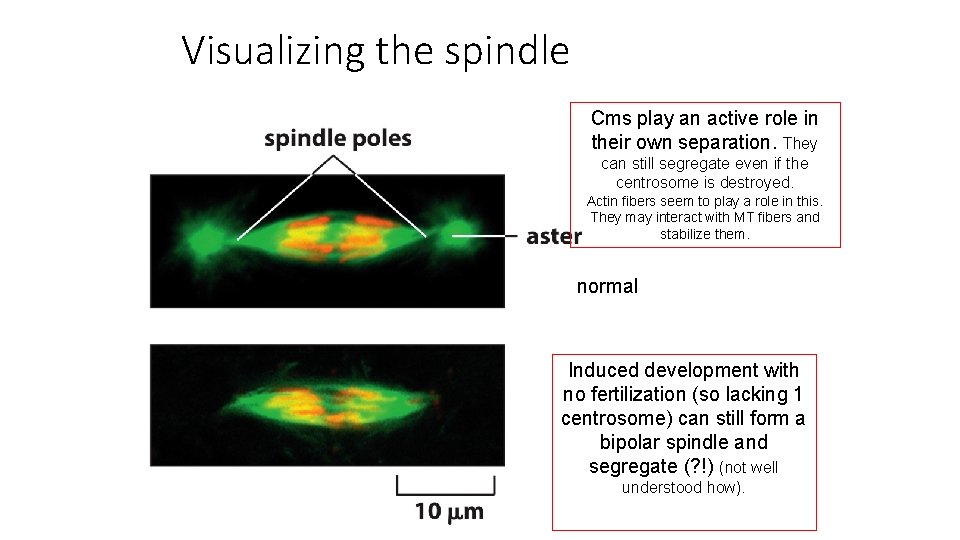
Visualizing the spindle Cms play an active role in their own separation. They can still segregate even if the centrosome is destroyed. Actin fibers seem to play a role in this. They may interact with MT fibers and stabilize them. normal Induced development with no fertilization (so lacking 1 centrosome) can still form a bipolar spindle and segregate (? !) (not well understood how).

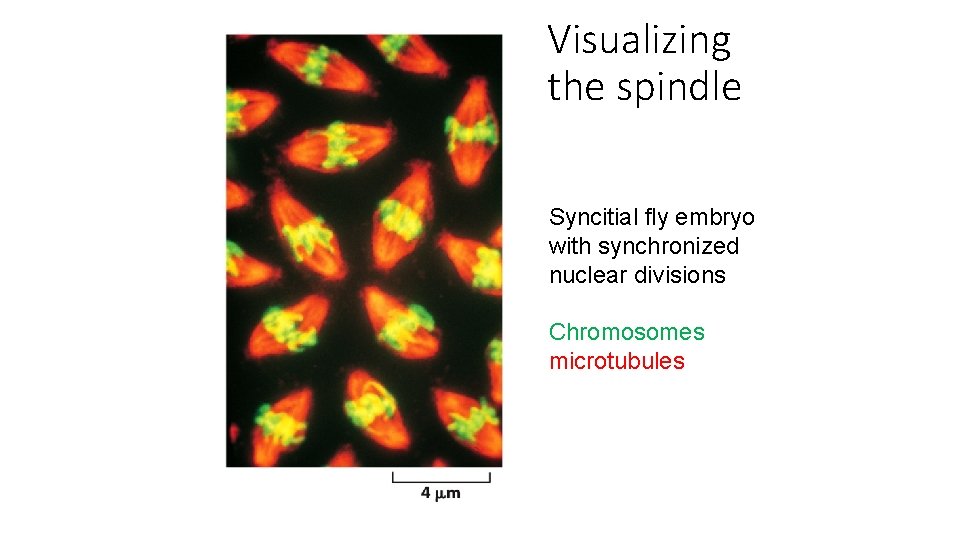
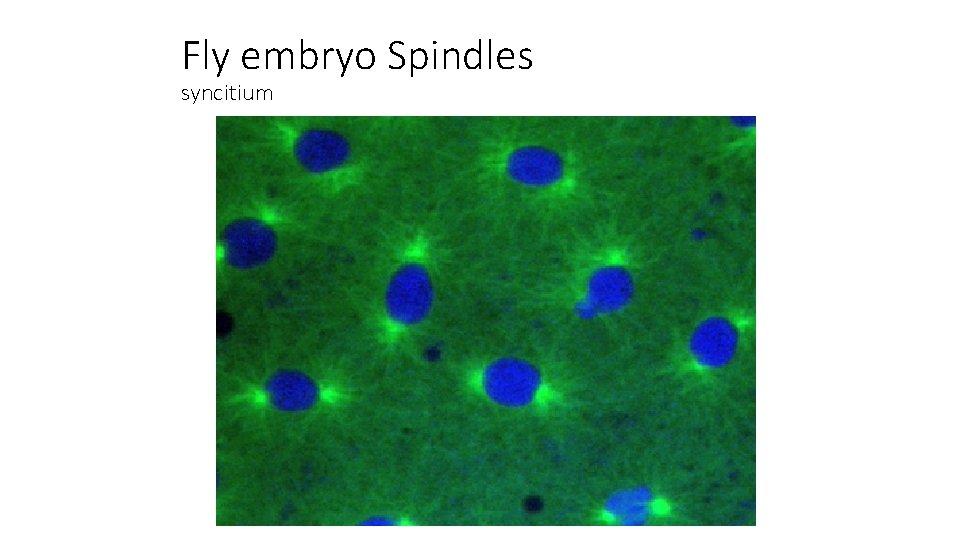
Visualizing the spindle Syncitial fly embryo with synchronized nuclear divisions Chromosomes microtubules

Fly embryo Spindles syncitium

Anaphase Separase (a protease) destroys cohesins. Normally inactivated by securin, but activation of APC inhibits securin, which activates separase destroying cohesins and thus promoting separation of chromatids in anaphase 2 driving forces 1 - anaphase A: MT shorten by depolymerization 2 - anaphase B: spindles move apart as cell elongates (by Cms separate at abt 1 µm/min. ratcheting of non-kinetochore MTs along each other)

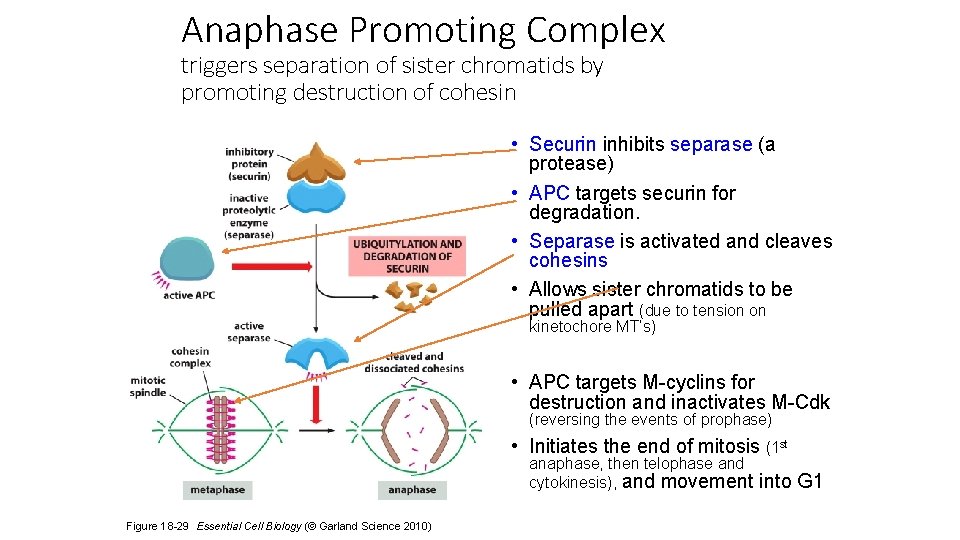
Anaphase Promoting Complex triggers separation of sister chromatids by promoting destruction of cohesin • Securin inhibits separase (a protease) • APC targets securin for degradation. • Separase is activated and cleaves cohesins • Allows sister chromatids to be pulled apart (due to tension on kinetochore MT’s) • APC targets M-cyclins for destruction and inactivates M-Cdk (reversing the events of prophase) • Initiates the end of mitosis (1 st anaphase, then telophase and cytokinesis), and movement Figure 18 -29 Essential Cell Biology (© Garland Science 2010) into G 1

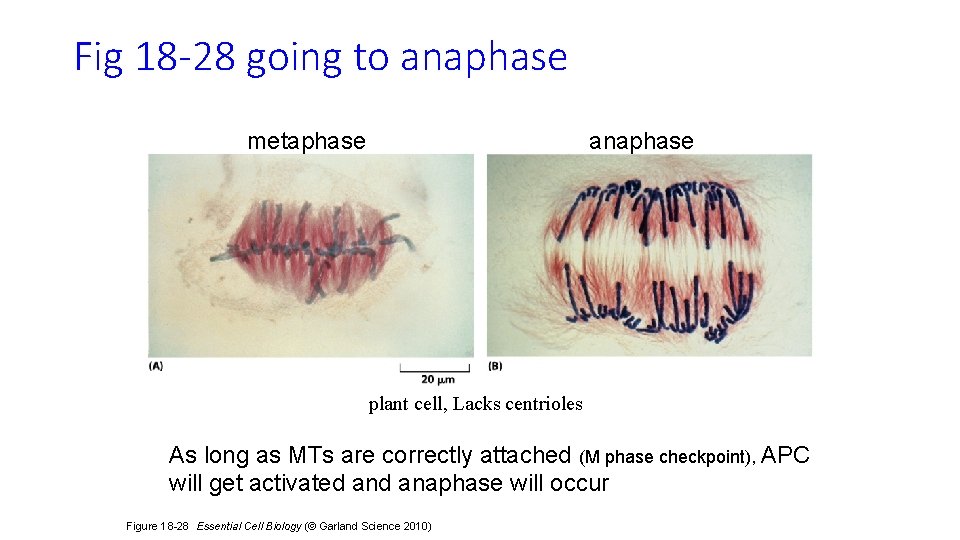
Fig 18 -28 going to anaphase metaphase anaphase plant cell, Lacks centrioles As long as MTs are correctly attached (M phase checkpoint), APC will get activated anaphase will occur Figure 18 -28 Essential Cell Biology (© Garland Science 2010)

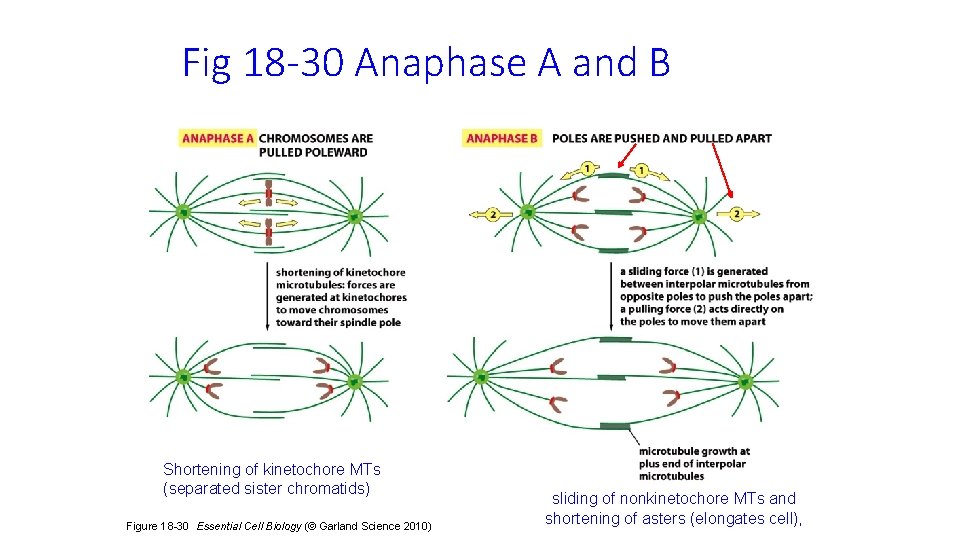
Fig 18 -30 Anaphase A and B Shortening of kinetochore MTs (separated sister chromatids) Figure 18 -30 Essential Cell Biology (© Garland Science 2010) sliding of nonkinetochore MTs and shortening of asters (elongates cell),

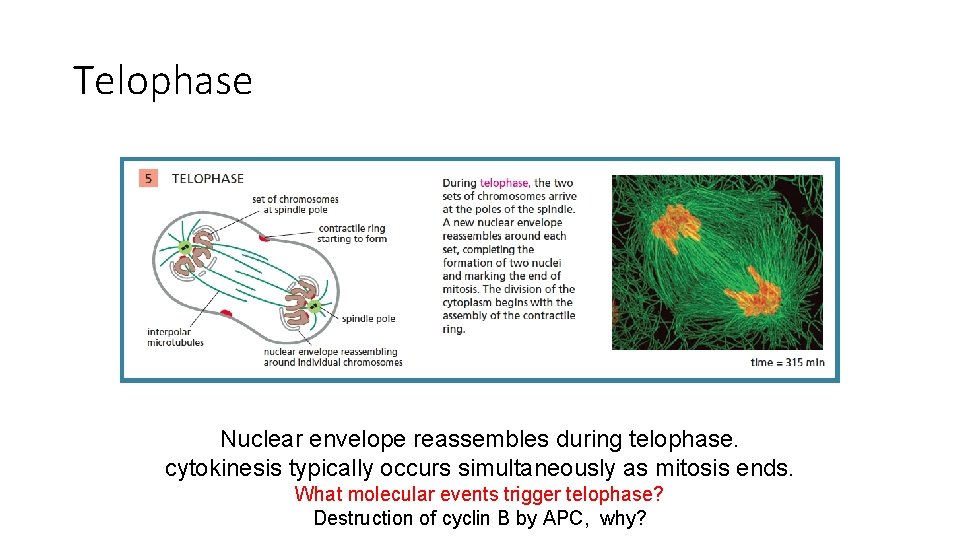
Telophase Nuclear envelope reassembles during telophase. cytokinesis typically occurs simultaneously as mitosis ends. What molecular events trigger telophase? Destruction of cyclin B by APC, why?

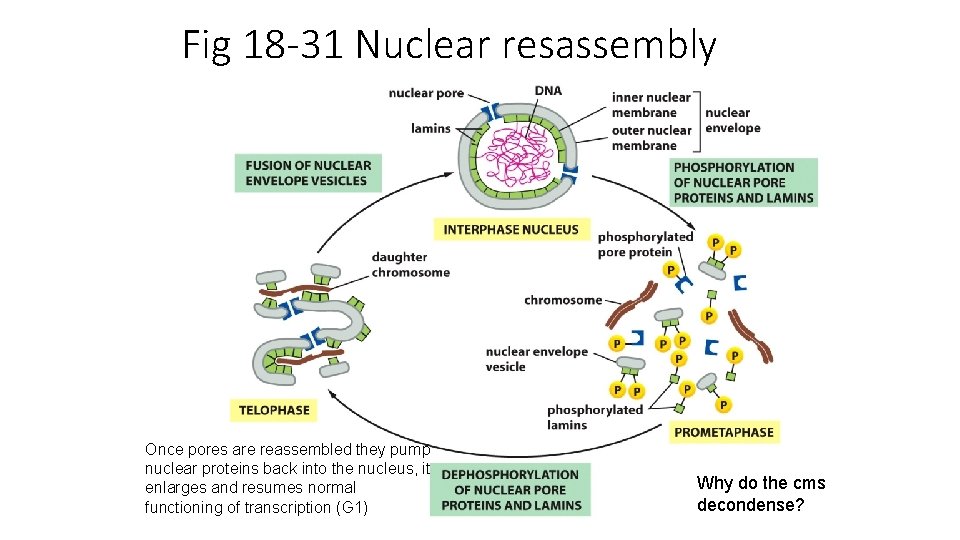
Fig 18 -31 Nuclear resassembly Once pores are reassembled they pump nuclear proteins back into the nucleus, it enlarges and resumes normal functioning of transcription (G 1) Why do the cms decondense?

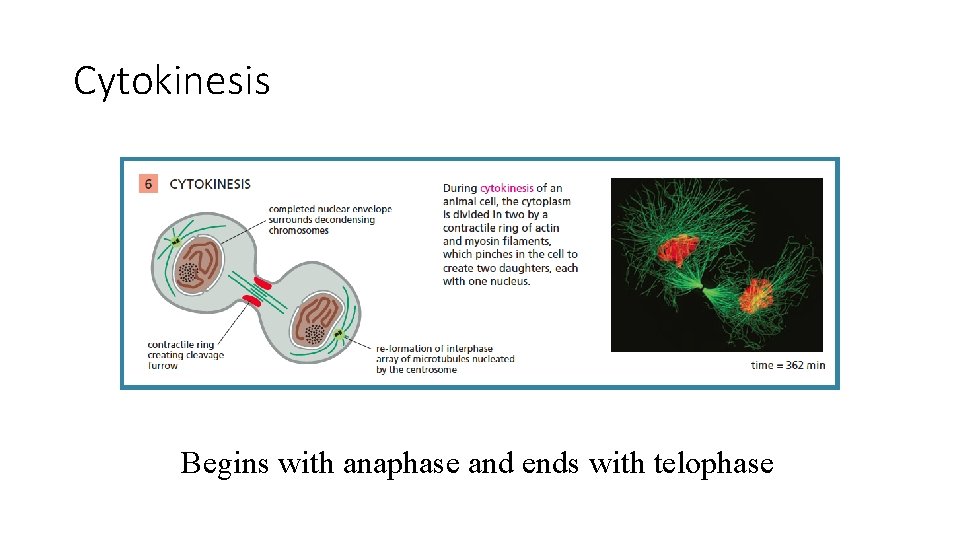
Cytokinesis Begins with anaphase and ends with telophase

The Mitotic Spindle Cytokinesis (in animals) is mediated by the contractile actin ring, but its formation is determined by the spindle apparatus Plane of cleavage Moving the spindle moves the cleavage plane, initially But later, cleavage will finish even if spindle is moved or destroyed

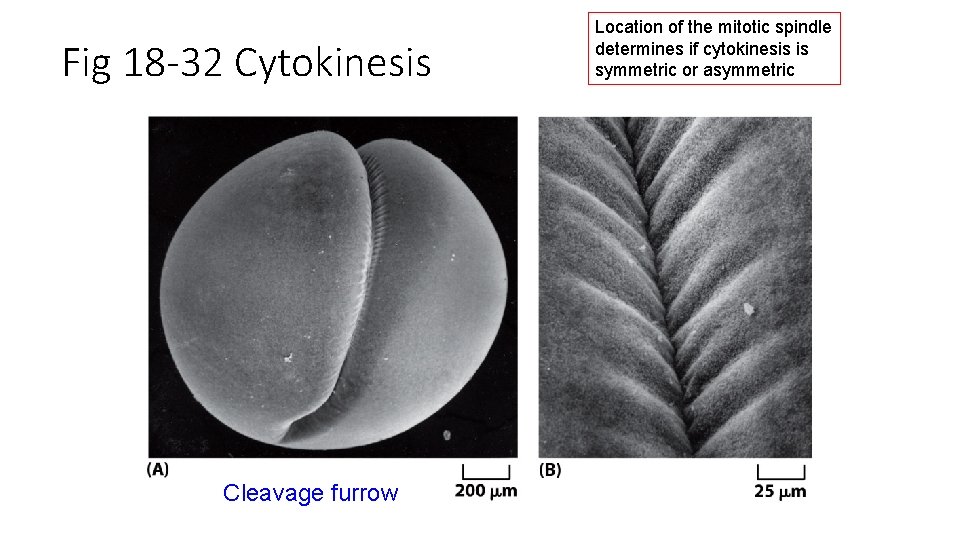
Fig 18 -32 Cytokinesis Cleavage furrow Location of the mitotic spindle determines if cytokinesis is symmetric or asymmetric

Fig 18 -33 The Contractile Ring

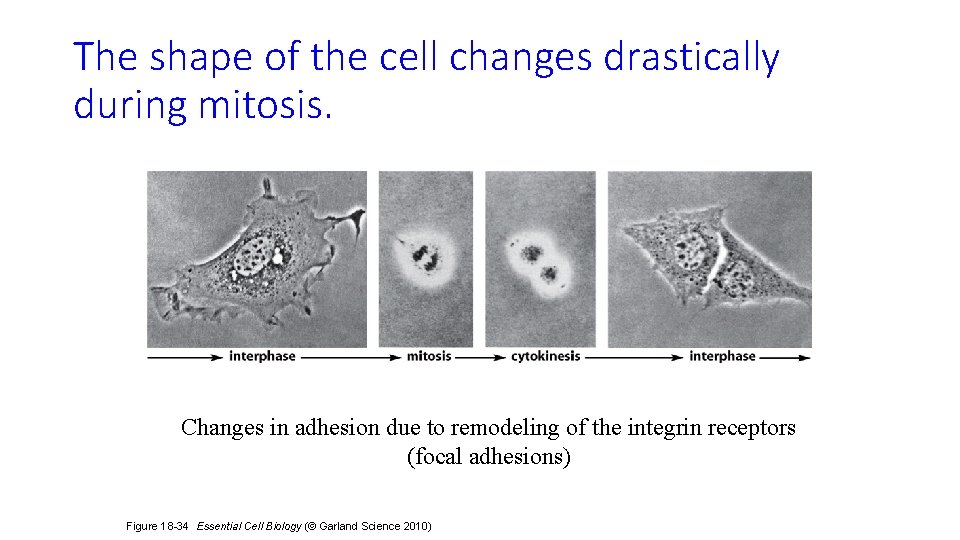
The shape of the cell changes drastically during mitosis. Changes in adhesion due to remodeling of the integrin receptors (focal adhesions) Figure 18 -34 Essential Cell Biology (© Garland Science 2010)

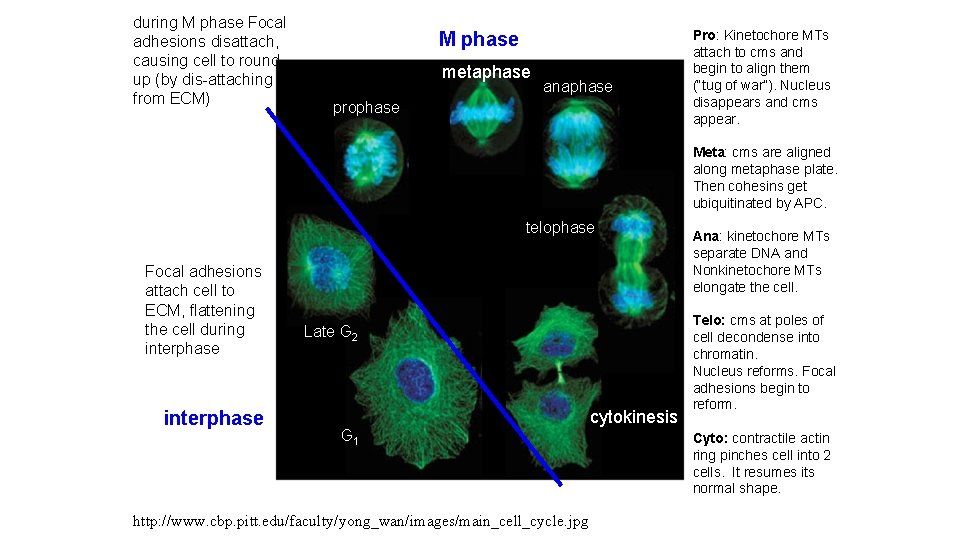
during M phase Focal adhesions disattach, causing cell to round up (by dis-attaching from ECM) M phase metaphase anaphase prophase Pro: Kinetochore MTs attach to cms and begin to align them (“tug of war”). Nucleus disappears and cms appear. Meta: cms are aligned along metaphase plate. Then cohesins get ubiquitinated by APC. telophase Focal adhesions attach cell to ECM, flattening the cell during interphase Late G 2 G 1 http: //www. cbp. pitt. edu/faculty/yong_wan/images/main_cell_cycle. jpg cytokinesis Ana: kinetochore MTs separate DNA and Nonkinetochore MTs elongate the cell. Telo: cms at poles of cell decondense into chromatin. Nucleus reforms. Focal adhesions begin to reform. Cyto: contractile actin ring pinches cell into 2 cells. It resumes its normal shape.

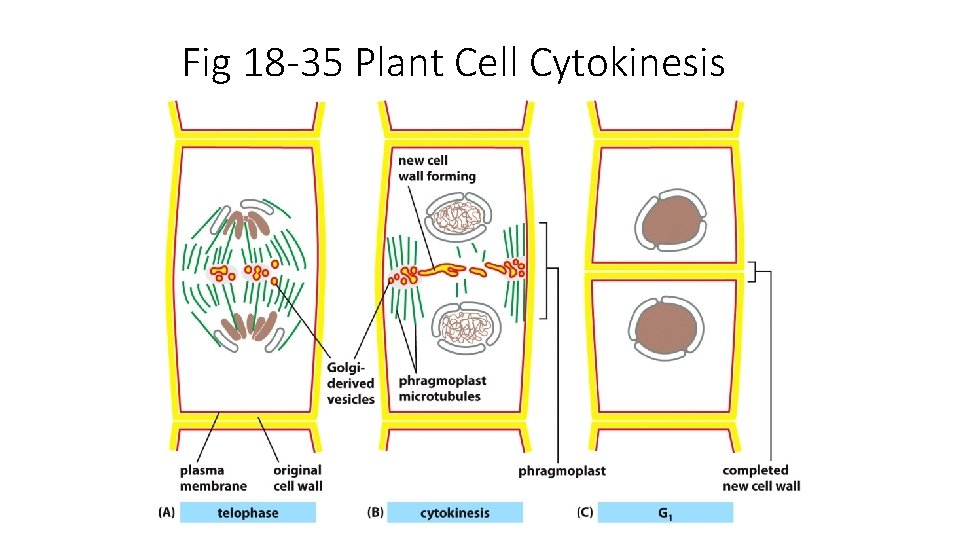
Fig 18 -35 Plant Cell Cytokinesis

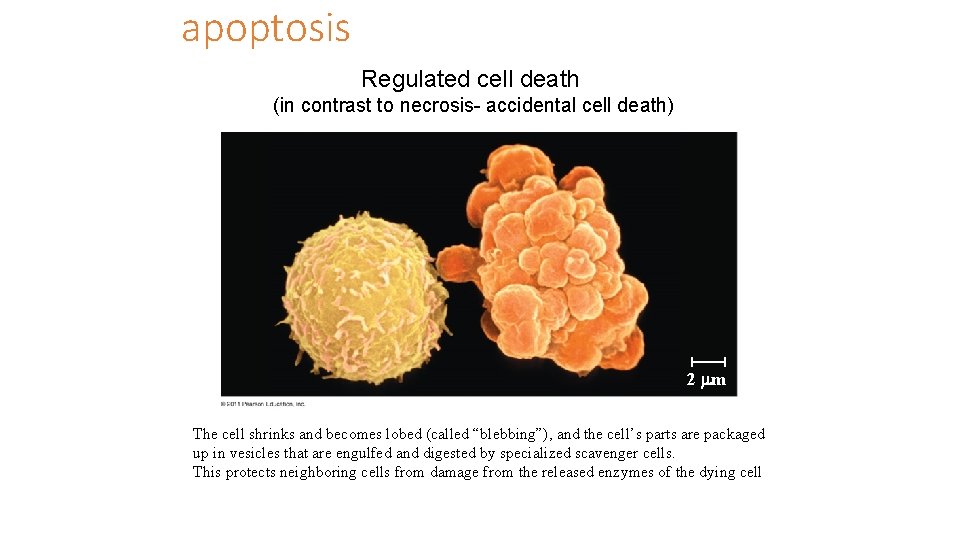
apoptosis Regulated cell death (in contrast to necrosis- accidental cell death) 2 m The cell shrinks and becomes lobed (called “blebbing”), and the cell’s parts are packaged up in vesicles that are engulfed and digested by specialized scavenger cells. This protects neighboring cells from damage from the released enzymes of the dying cell

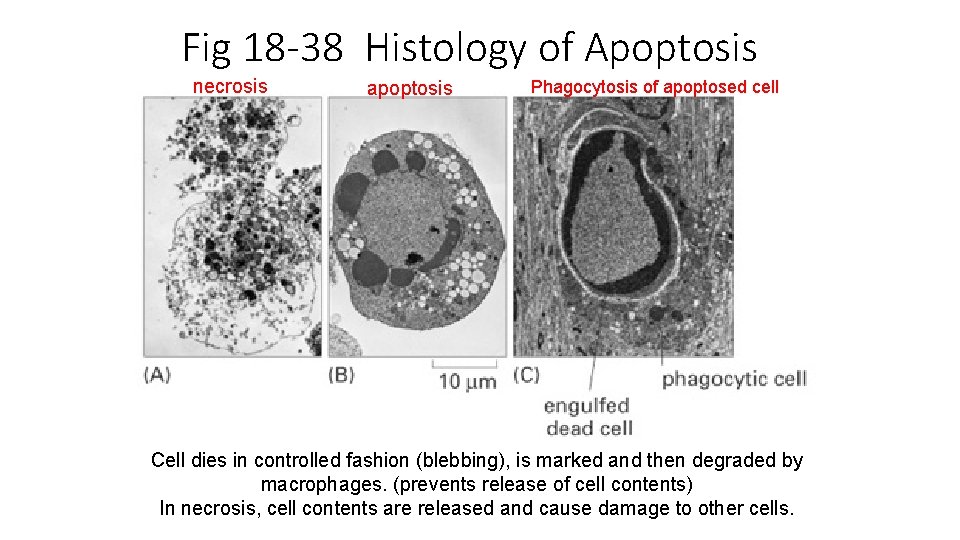
Fig 18 -38 Histology of Apoptosis necrosis apoptosis Phagocytosis of apoptosed cell Cell dies in controlled fashion (blebbing), is marked and then degraded by macrophages. (prevents release of cell contents) In necrosis, cell contents are released and cause damage to other cells.

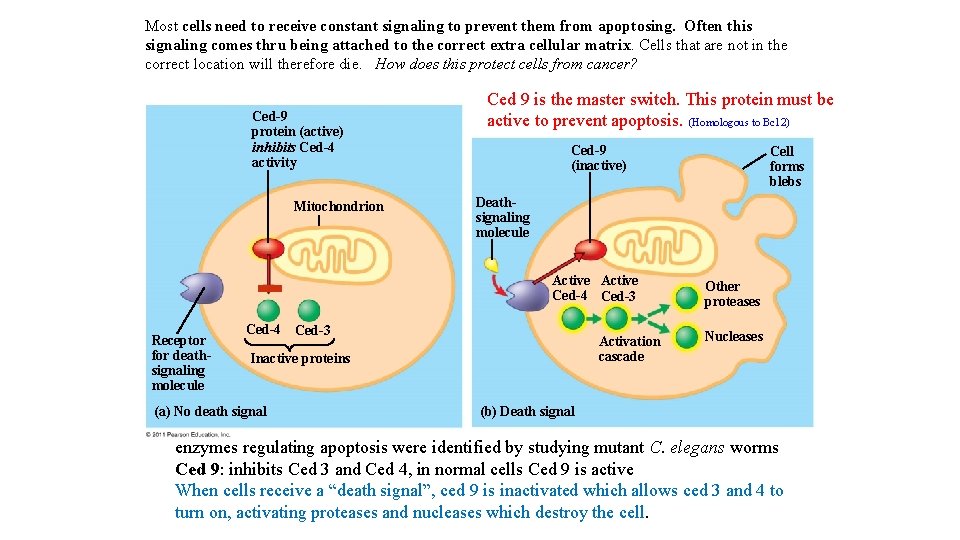
Most cells need to receive constant signaling to prevent them from apoptosing. Often this signaling comes thru being attached to the correct extra cellular matrix. Cells that are not in the correct location will therefore die. How does this protect cells from cancer? Ced-9 protein (active) inhibits Ced-4 activity Mitochondrion Ced 9 is the master switch. This protein must be active to prevent apoptosis. (Homologous to Bc 12) Ced-9 (inactive) Deathsignaling molecule Active Ced-4 Ced-3 Receptor for deathsignaling molecule Ced-4 Ced-3 Activation cascade Inactive proteins (a) No death signal Cell forms blebs Other proteases Nucleases (b) Death signal enzymes regulating apoptosis were identified by studying mutant C. elegans worms Ced 9: inhibits Ced 3 and Ced 4, in normal cells Ced 9 is active When cells receive a “death signal”, ced 9 is inactivated which allows ced 3 and 4 to turn on, activating proteases and nucleases which destroy the cell.

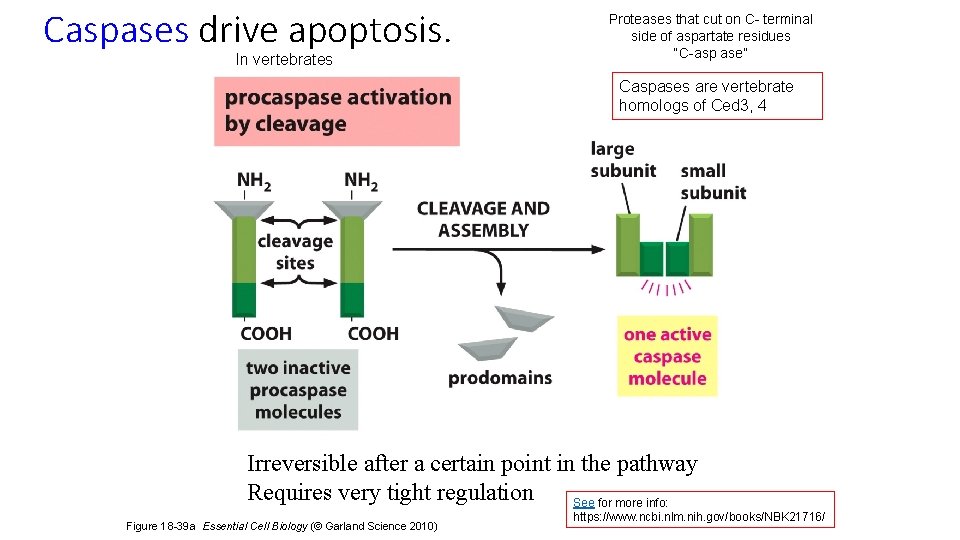
Caspases drive apoptosis. In vertebrates Proteases that cut on C- terminal side of aspartate residues “C-asp ase” Caspases are vertebrate homologs of Ced 3, 4 Irreversible after a certain point in the pathway Requires very tight regulation See for more info: Figure 18 -39 a Essential Cell Biology (© Garland Science 2010) https: //www. ncbi. nlm. nih. gov/books/NBK 21716/

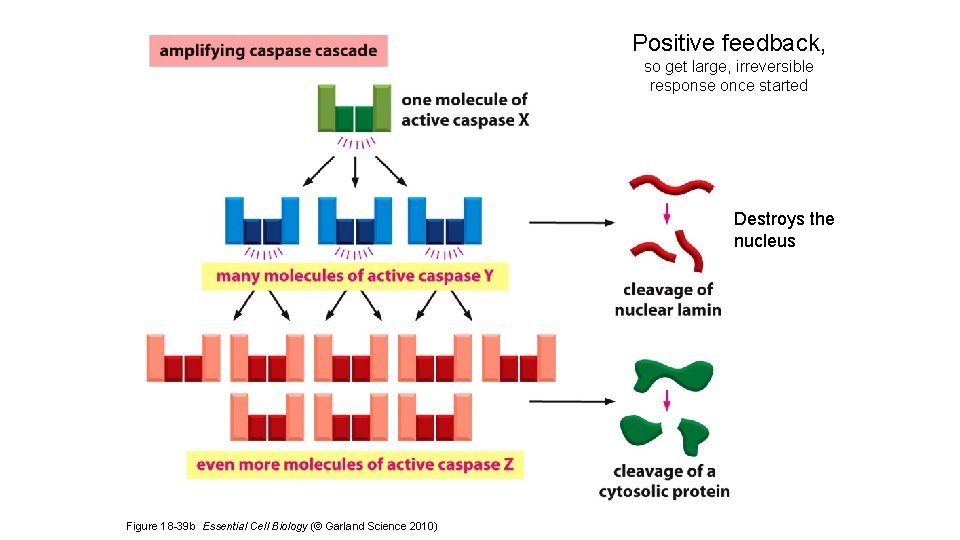
Positive feedback, so get large, irreversible response once started Destroys the nucleus Figure 18 -39 b Essential Cell Biology (© Garland Science 2010)

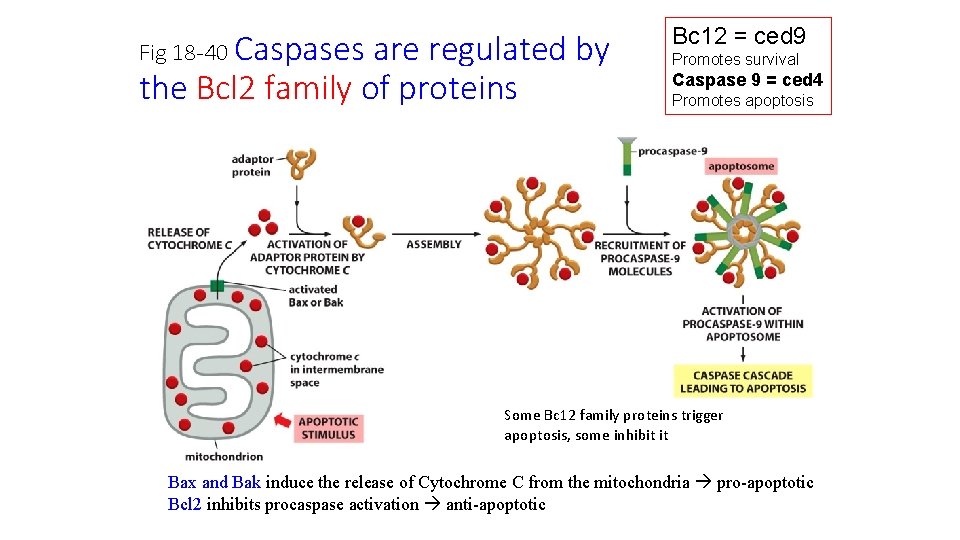
Fig 18 -40 Caspases are regulated by the Bcl 2 family of proteins Bc 12 = ced 9 Promotes survival Caspase 9 = ced 4 Promotes apoptosis Some Bc 12 family proteins trigger apoptosis, some inhibit it Bax and Bak induce the release of Cytochrome C from the mitochondria pro-apoptotic Bcl 2 inhibits procaspase activation anti-apoptotic

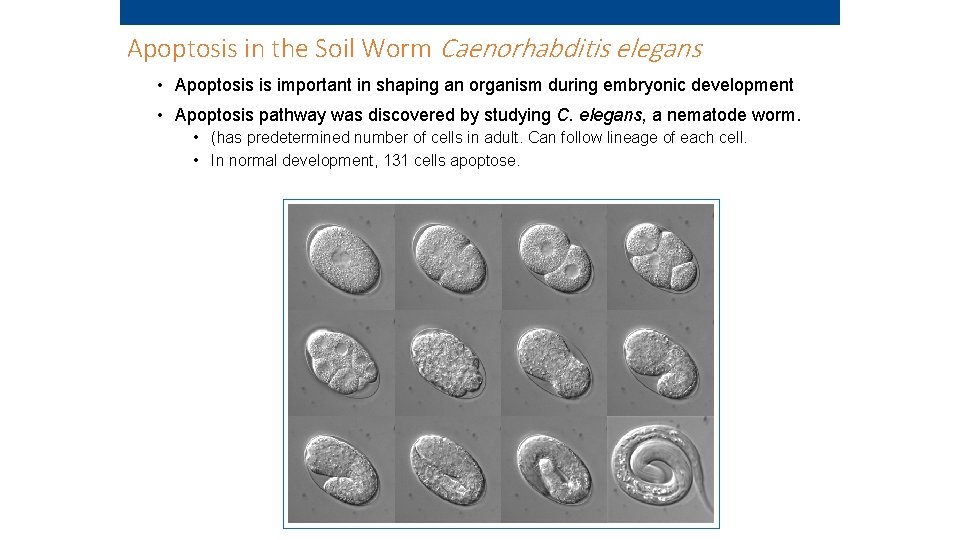
Apoptosis in the Soil Worm Caenorhabditis elegans • Apoptosis is important in shaping an organism during embryonic development • Apoptosis pathway was discovered by studying C. elegans, a nematode worm. • (has predetermined number of cells in adult. Can follow lineage of each cell. • In normal development, 131 cells apoptose.

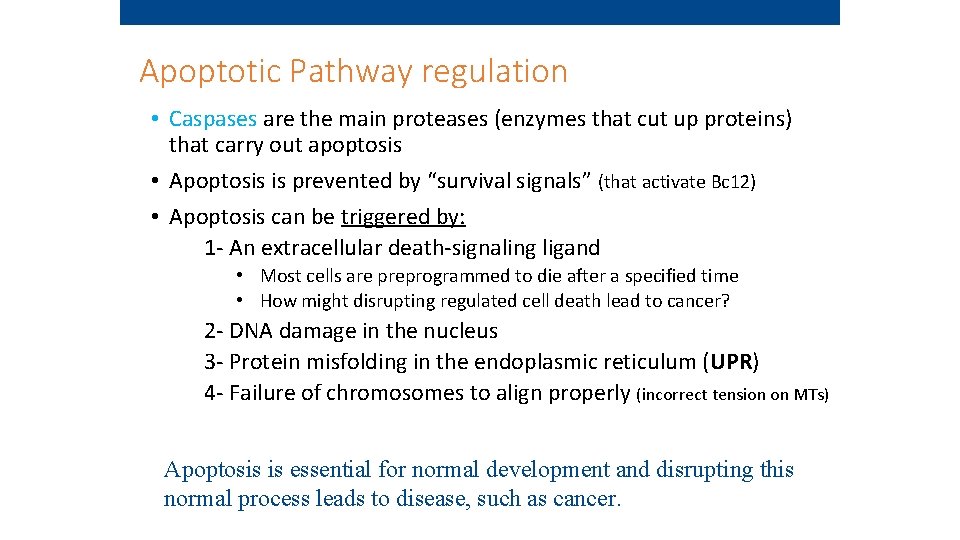
Apoptotic Pathway regulation • Caspases are the main proteases (enzymes that cut up proteins) that carry out apoptosis • Apoptosis is prevented by “survival signals” (that activate Bc 12) • Apoptosis can be triggered by: 1 - An extracellular death-signaling ligand • Most cells are preprogrammed to die after a specified time • How might disrupting regulated cell death lead to cancer? 2 - DNA damage in the nucleus 3 - Protein misfolding in the endoplasmic reticulum (UPR) 4 - Failure of chromosomes to align properly (incorrect tension on MTs) Apoptosis is essential for normal development and disrupting this normal process leads to disease, such as cancer.

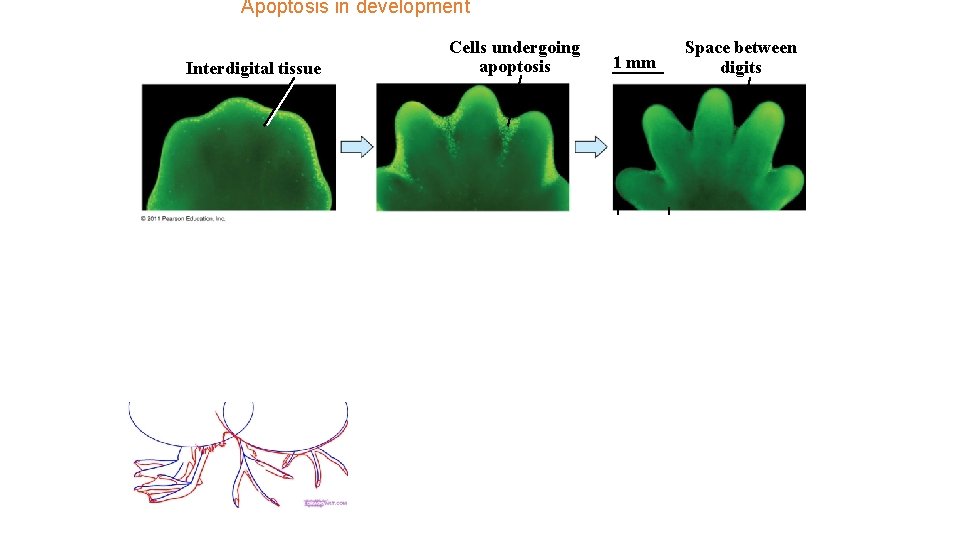
Apoptosis in development Interdigital tissue Cells undergoing apoptosis syndactyly 1 mm Space between digits polydactyly

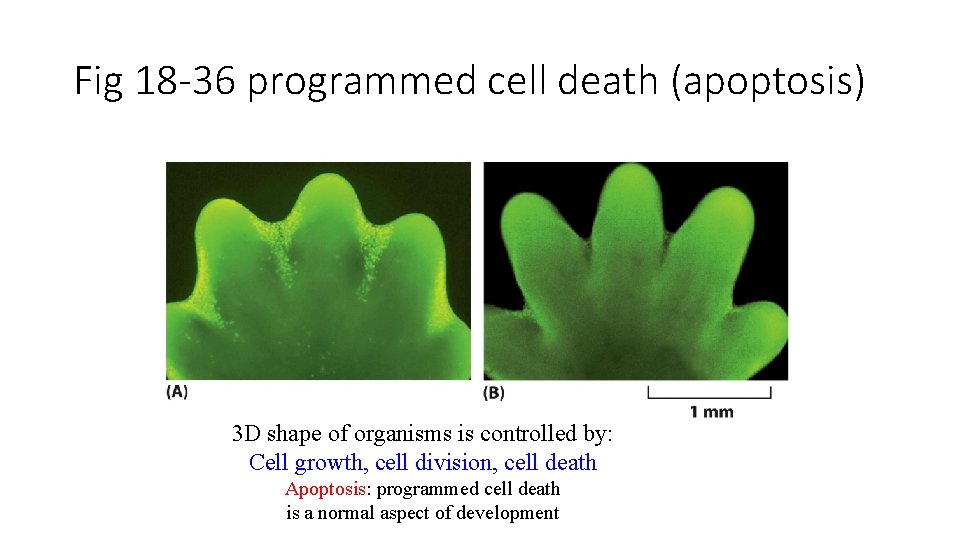
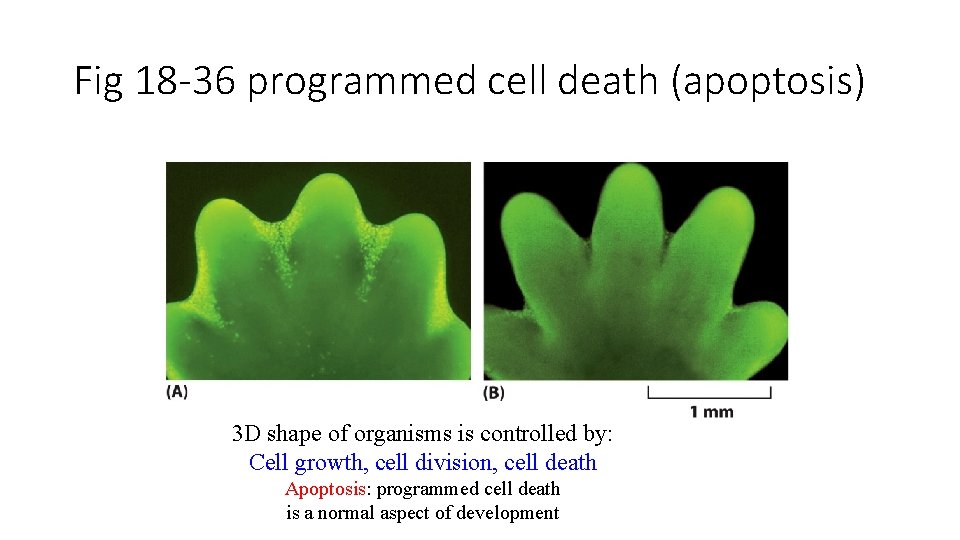
Fig 18 -36 programmed cell death (apoptosis) 3 D shape of organisms is controlled by: Cell growth, cell division, cell death Apoptosis: programmed cell death is a normal aspect of development

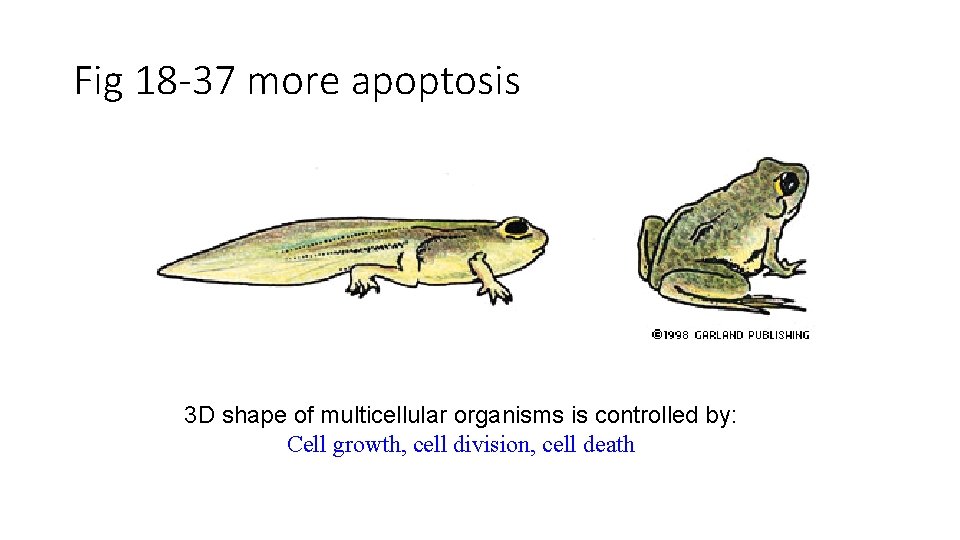
Fig 18 -37 more apoptosis 3 D shape of multicellular organisms is controlled by: Cell growth, cell division, cell death

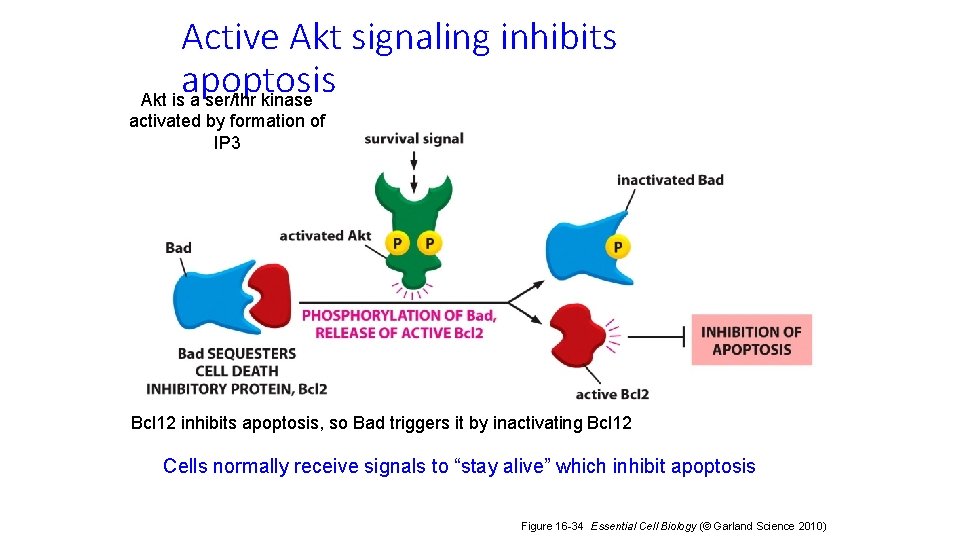
Active Akt signaling inhibits apoptosis Akt is a ser/thr kinase activated by formation of IP 3 Bcl 12 inhibits apoptosis, so Bad triggers it by inactivating Bcl 12 Cells normally receive signals to “stay alive” which inhibit apoptosis Figure 16 -34 Essential Cell Biology (© Garland Science 2010)

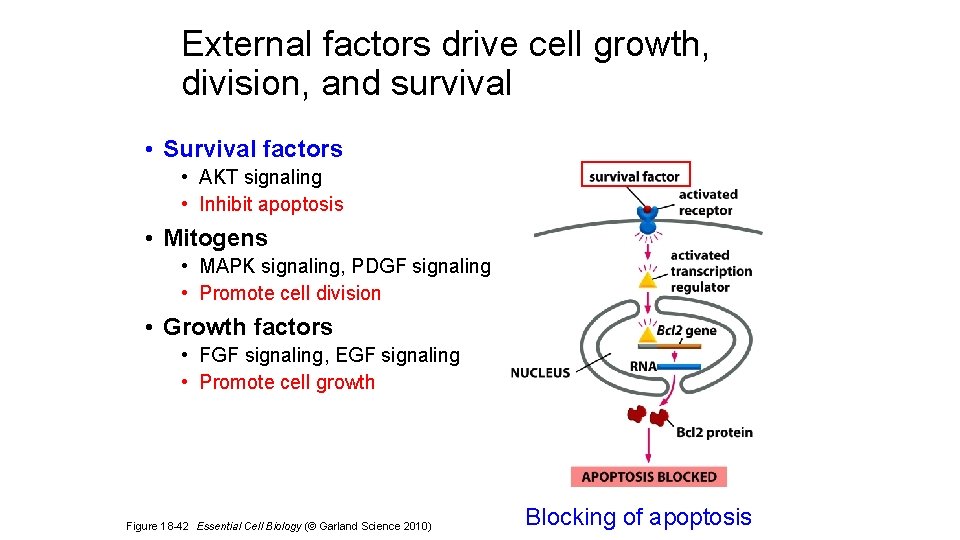
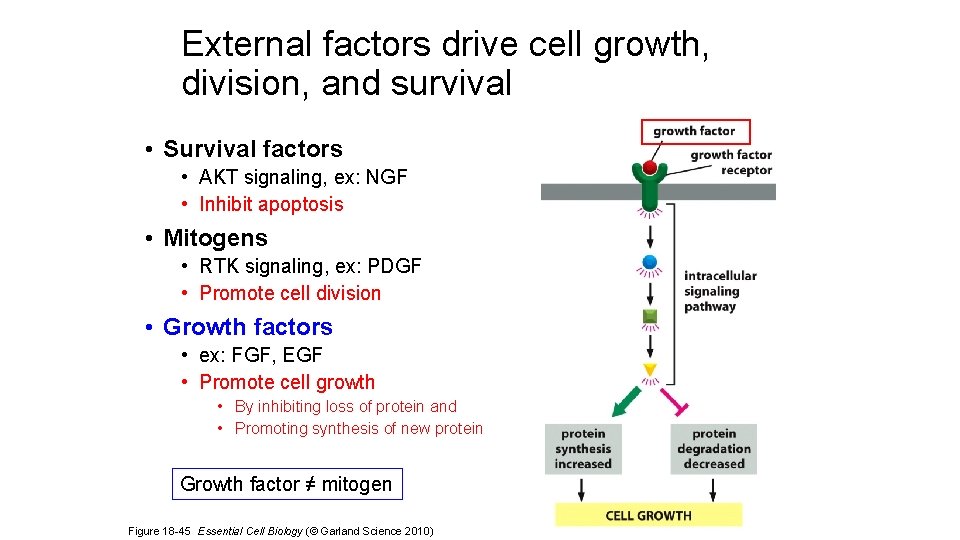
External factors drive cell growth, division, and survival • Survival factors • AKT signaling • Inhibit apoptosis • Mitogens • MAPK signaling, PDGF signaling • Promote cell division • Growth factors • FGF signaling, EGF signaling • Promote cell growth Figure 18 -42 Essential Cell Biology (© Garland Science 2010) Blocking of apoptosis

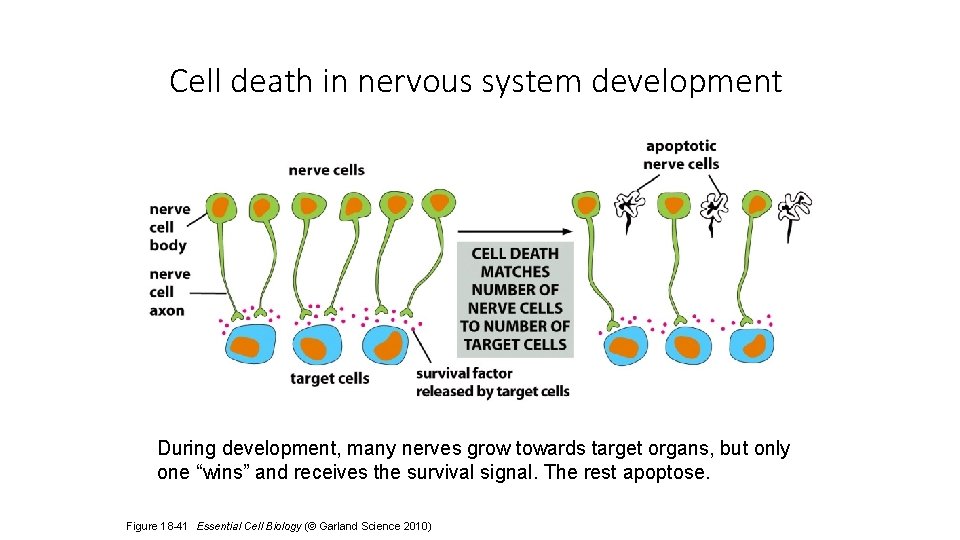
Cell death in nervous system development During development, many nerves grow towards target organs, but only one “wins” and receives the survival signal. The rest apoptose. Figure 18 -41 Essential Cell Biology (© Garland Science 2010)

External factors drive cell growth, division, and survival • Survival factors • AKT signaling, ex: NGF • Inhibit apoptosis • Mitogens • RTK signaling, ex: PDGF • Promote cell division • Growth factors • ex: FGF, EGF • Promote cell growth • By inhibiting loss of protein and • Promoting synthesis of new protein Growth factor ≠ mitogen Figure 18 -45 Essential Cell Biology (© Garland Science 2010)

Cell culture media contains a source of growth factors and mitogens Calf serum BSA (makes tissue culture possible since contains important growth factors. Also useful to block “sticky sites” in immunohistochemistry) Figure 18 -44 Essential Cell Biology (© Garland Science 2010)

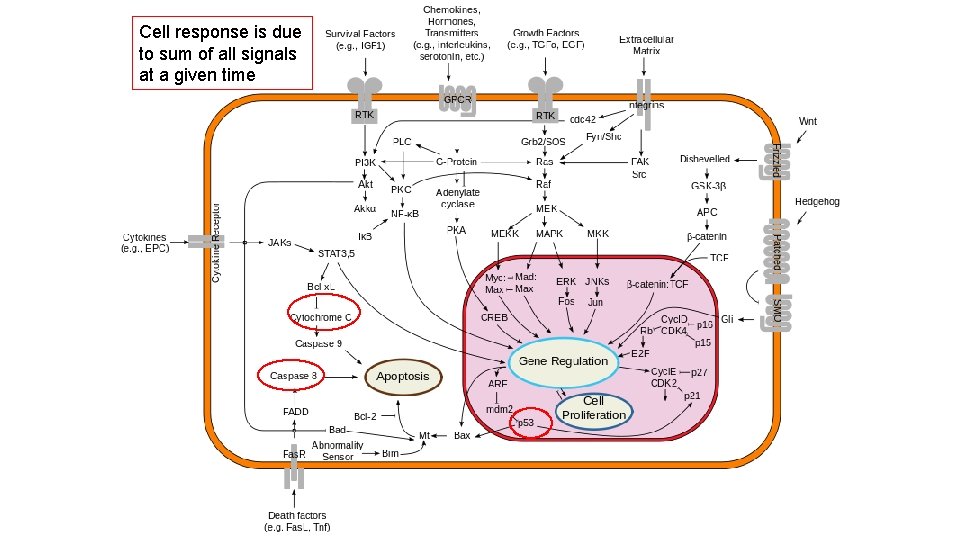
Cell response is due to sum of all signals at a given time

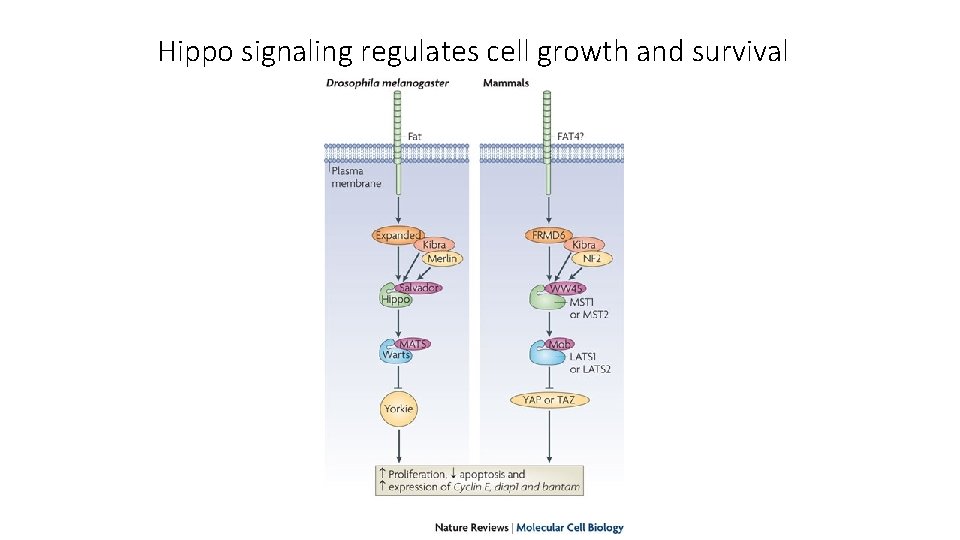
Hippo signaling regulates cell growth and survival

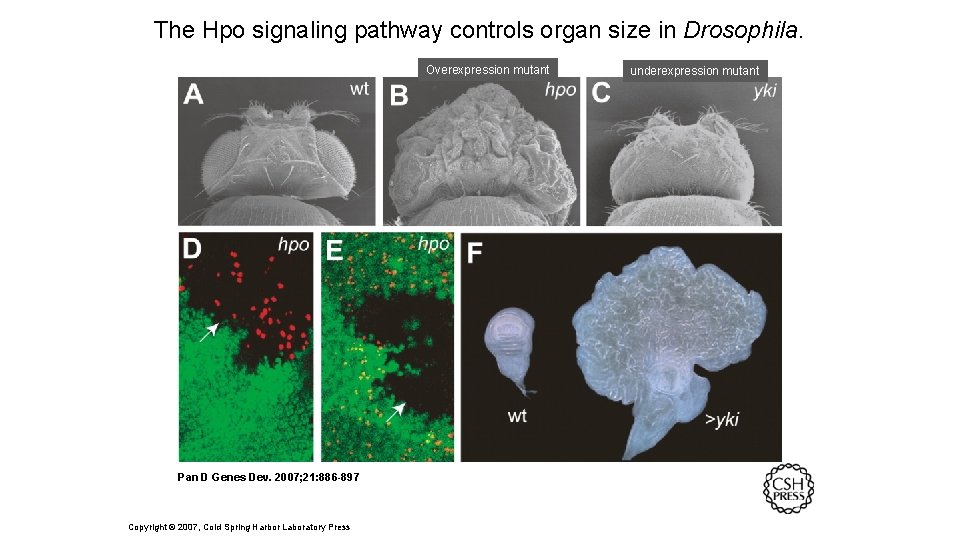
The Hpo signaling pathway controls organ size in Drosophila. Overexpression mutant Pan D Genes Dev. 2007; 21: 886 -897 Copyright © 2007, Cold Spring Harbor Laboratory Press underexpression mutant

Slides not used

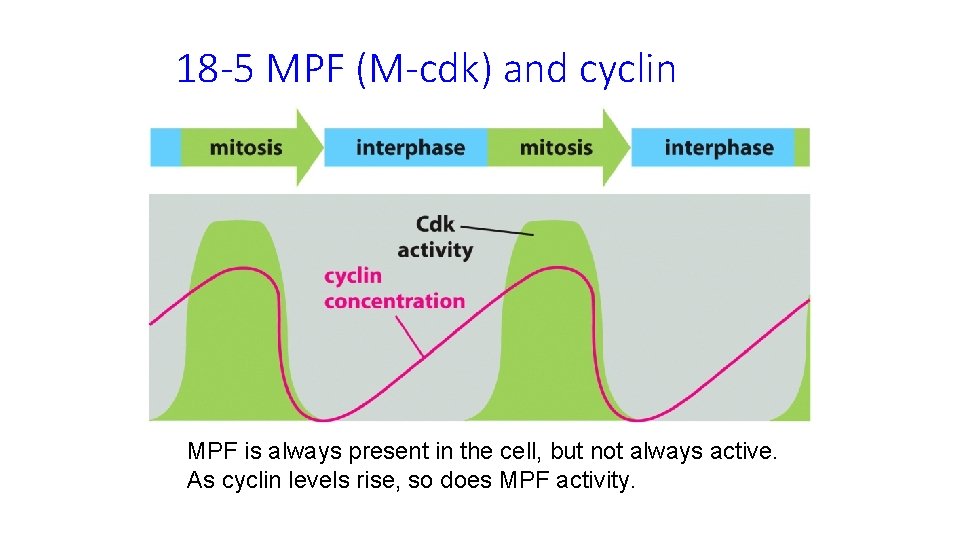
18 -5 MPF (M-cdk) and cyclin MPF is always present in the cell, but not always active. As cyclin levels rise, so does MPF activity.

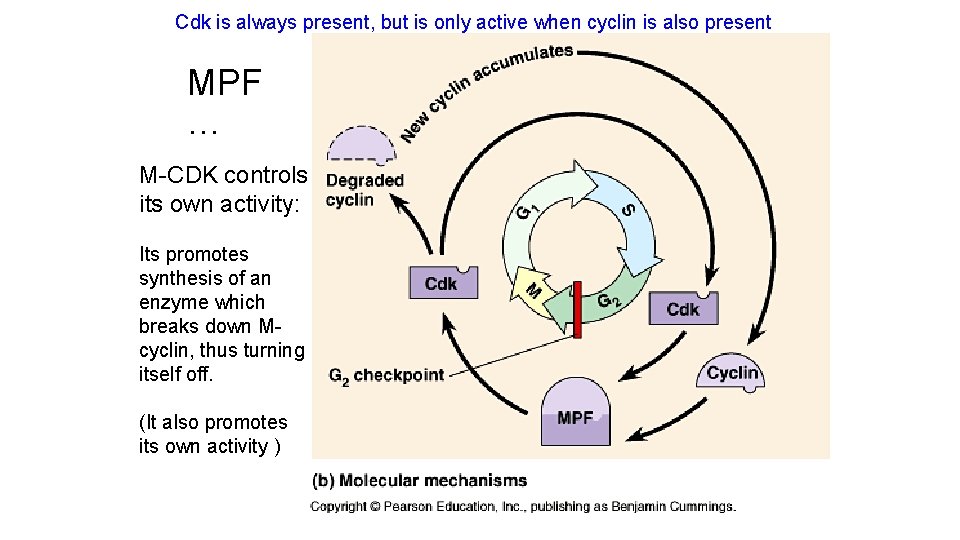
Cdk is always present, but is only active when cyclin is also present MPF … M-CDK controls its own activity: Its promotes synthesis of an enzyme which breaks down Mcyclin, thus turning itself off. (It also promotes its own activity )

Fig 18 -36 programmed cell death (apoptosis) 3 D shape of organisms is controlled by: Cell growth, cell division, cell death Apoptosis: programmed cell death is a normal aspect of development