Changes of State Chapter 4 2 Changes of







- Slides: 14

Changes of State Chapter 4 -2

Changes of State • A change of state is the conversion of a substance from one physical form to another. • All the changes are physical changes.

• The identity of the substance does not change.

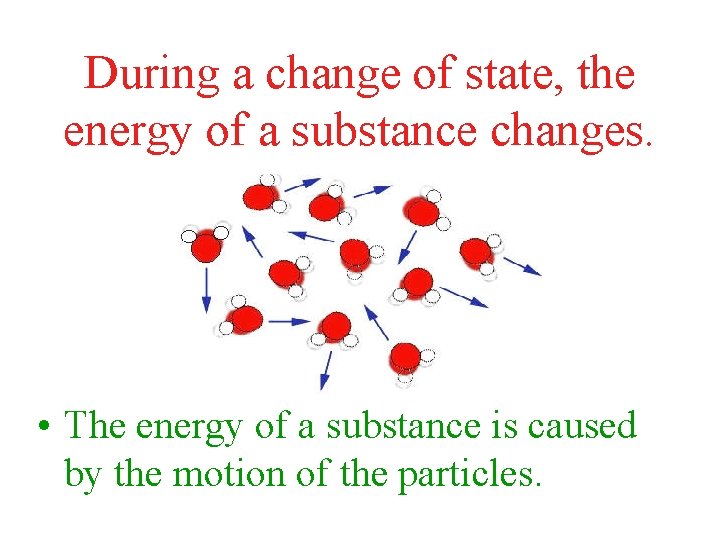
During a change of state, the energy of a substance changes. • The energy of a substance is caused by the motion of the particles.

MELTING: Solid to Liquid • Melting point is the temperature at which a substance melts. • The melting point is different for all substances. • For a solid to melt, energy must be absorbed to make the molecules move faster.

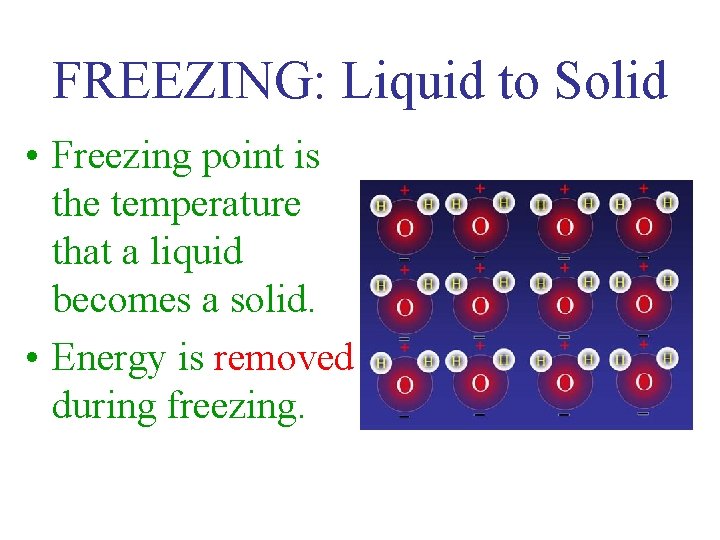
FREEZING: Liquid to Solid • Freezing point is the temperature that a liquid becomes a solid. • Energy is removed during freezing.

EVAPORATION: Liquid to Gas • Evaporation is the change of state from liquid to a gas. • It happens at the surface. • Sweat evaporating cools your body.

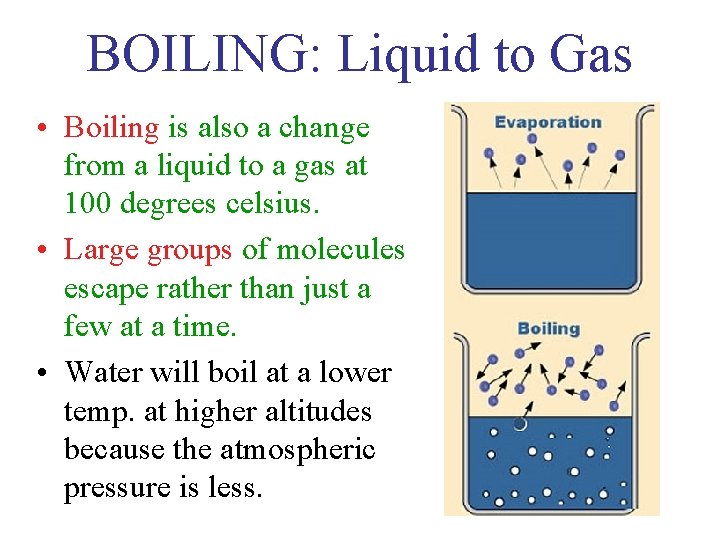
BOILING: Liquid to Gas • Boiling is also a change from a liquid to a gas at 100 degrees celsius. • Large groups of molecules escape rather than just a few at a time. • Water will boil at a lower temp. at higher altitudes because the atmospheric pressure is less.

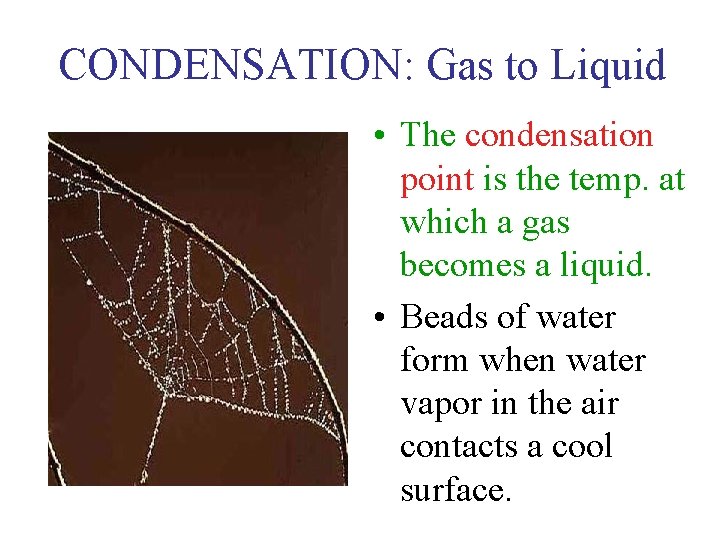
CONDENSATION: Gas to Liquid • The condensation point is the temp. at which a gas becomes a liquid. • Beads of water form when water vapor in the air contacts a cool surface.

SUBLIMATION: Solid to Gas • Sublimation is the change of state in which a solid changes directly to a gas. • Dry ice goes through sublimation instead of melting into a liquid.

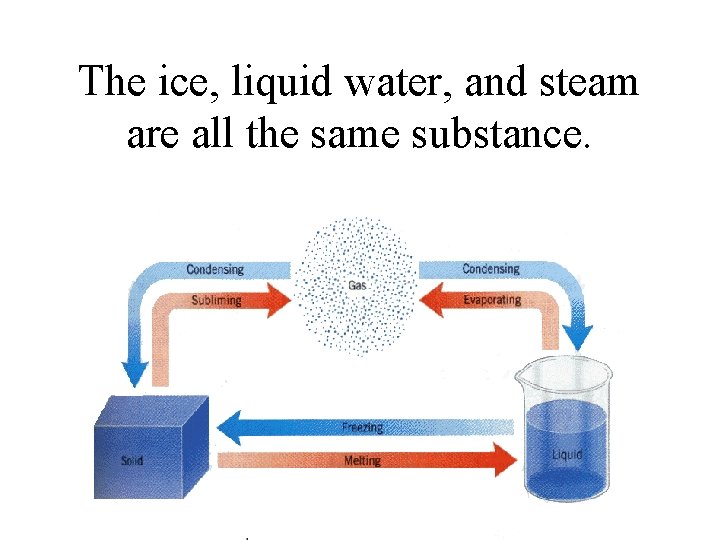
The ice, liquid water, and steam are all the same substance.

Temperature and Changes of State • When substances gain or lose energy, its temp. changes or its state changes. • When the temp. changes so does the particle movement. • Higher temp. = faster movement • Lower temp. = slower movement

The temperature remains the same at the horizontal lines while energy is added constantly.

Stop here and do chart.