Changes of State Chapter 3 Section 3 Changing

- Slides: 10

Changes of State Chapter 3 Section 3

Changing States • Caused By: removing or adding thermal energy a substance can lose or absorb thermal energy its temperature changes the speed of its particles changes and the state changes

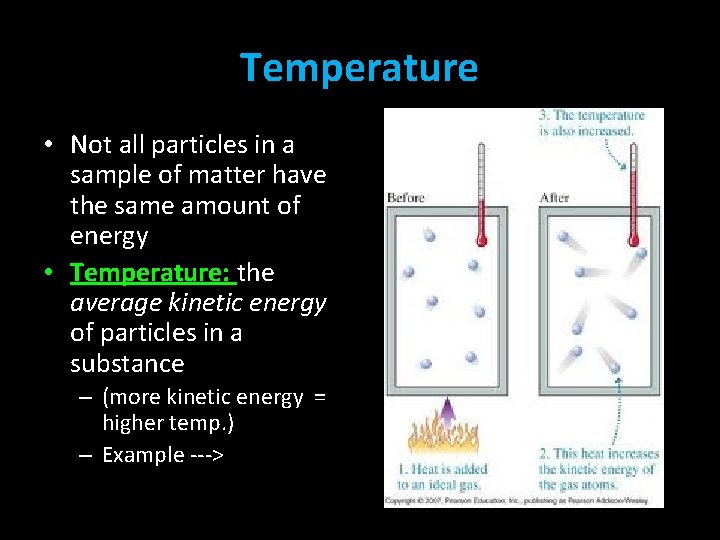
Temperature • Not all particles in a sample of matter have the same amount of energy • Temperature: the average kinetic energy of particles in a substance – (more kinetic energy = higher temp. ) – Example --->

Thermal Energy & Heat • Thermal Energy – Is the total energy of the particles in a substance • Heat – movement of thermal energy from a warmer substance to cooler one (always w → c) – heating → • ↑ thermal energy • ↑ motion of particles • ↑ temp

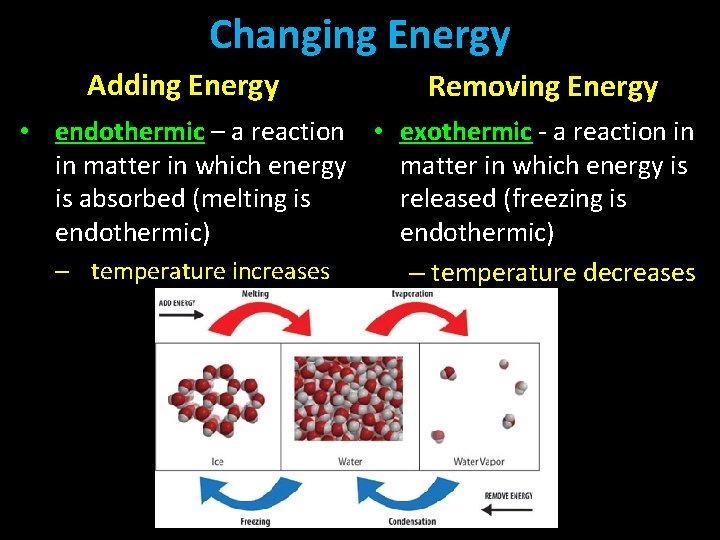
Changing Energy Adding Energy Removing Energy • endothermic – a reaction • exothermic - a reaction in in matter in which energy is is absorbed (melting is released (freezing is endothermic) – temperature increases – temperature decreases

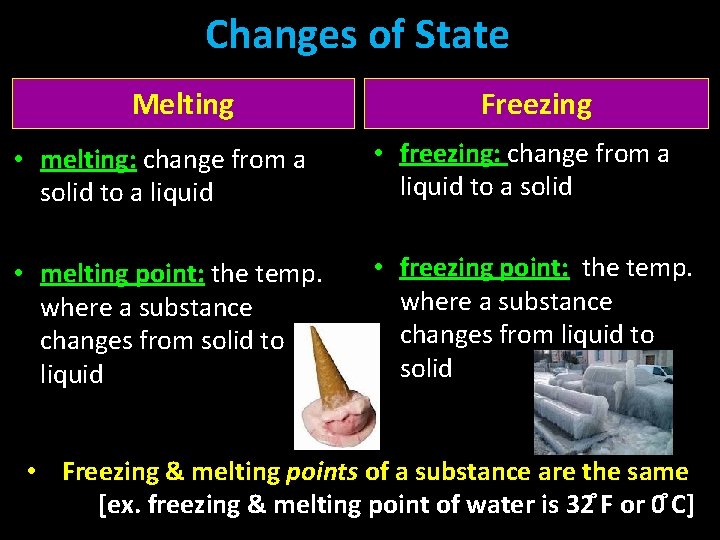
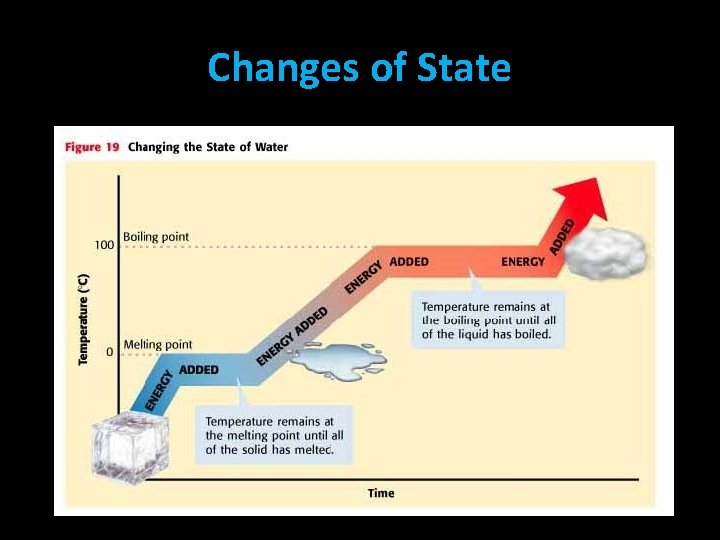
Changes of State Melting Freezing • melting: change from a solid to a liquid • freezing: change from a liquid to a solid • melting point: the temp. where a substance changes from solid to liquid • freezing point: the temp. where a substance changes from liquid to solid • Freezing & melting points of a substance are the same [ex. freezing & melting point of water is 32 F or 0 C]

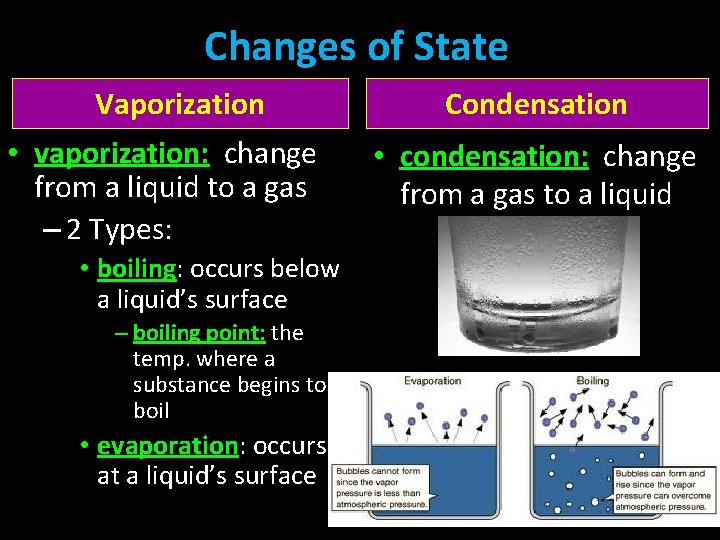
Changes of State Vaporization • vaporization: change from a liquid to a gas – 2 Types: • boiling: occurs below a liquid’s surface – boiling point: the temp. where a substance begins to boil • evaporation: occurs at a liquid’s surface Condensation • condensation: change from a gas to a liquid

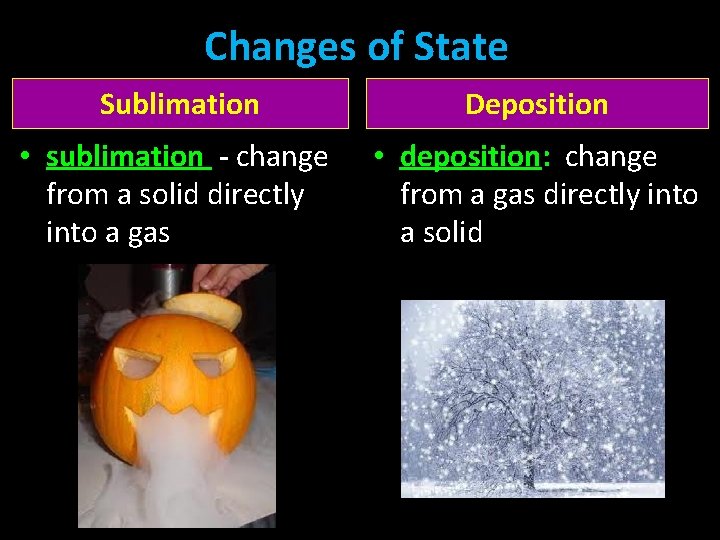
Changes of State Sublimation Deposition • sublimation - change from a solid directly into a gas • deposition: change from a gas directly into a solid

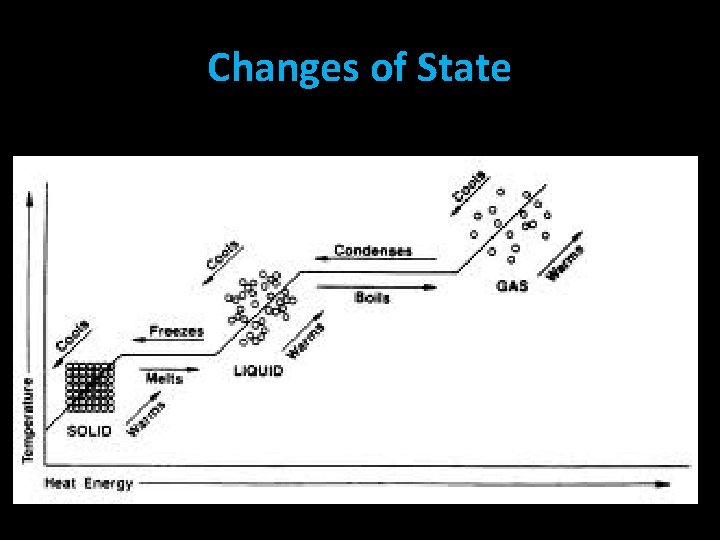
Changes of State

Changes of State