Changes noted on this slide Slide count includes






























- Slides: 45

Changes noted on this slide. Slide count includes this new slide. Slide 6: Argue moved from 1 st in list to middle of list. Slide 8: Put Creativity into list. Which two skills are you referring to in the notes? Slide 10: Added “repeatable by others” to individual, dynamic and messy. I think that is the key difference between science and arts, science and theology. Slide 13: fixed spelling of “discussion” Slide 15: Should one of these be “ 603? ” Slide 19: piechart changed to “scholarship” Slide 20: clarified Points for Exams Slide 23: Spelled out Misc - Added piechart for summary discussion of grades and advice regarding successful approach. Slide 33: Link to NAS document explaining scientific method added Slide 36: picture of Dalton added Slides 37 -38: inserted two slides since one of Dalton’s Laws mentioned here (and in book) is incorrect. A great chance to bring up the fact that “Laws” are not immutable. Slide 44: space added

Intersection 1: Intro to Gateway 9/5/06 Reading: 1. 1 -1. 2 p 2 -5


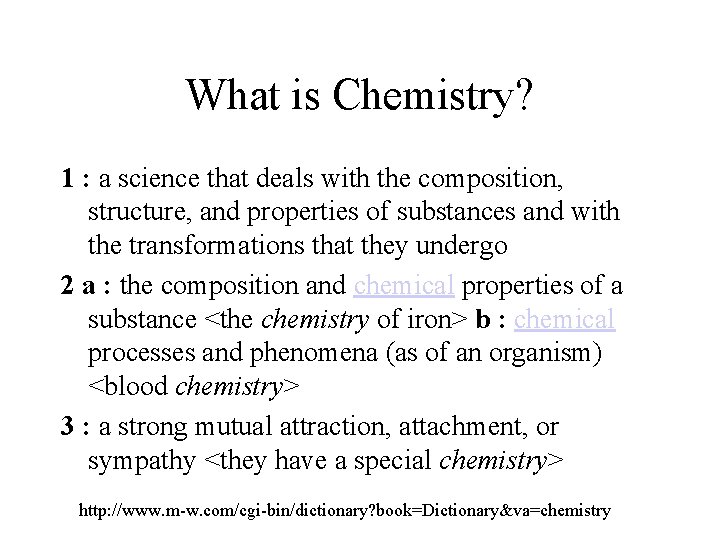
What is Chemistry? 1 : a science that deals with the composition, structure, and properties of substances and with the transformations that they undergo 2 a : the composition and chemical properties of a substance <the chemistry of iron> b : chemical processes and phenomena (as of an organism) <blood chemistry> 3 : a strong mutual attraction, attachment, or sympathy <they have a special chemistry> http: //www. m-w. com/cgi-bin/dictionary? book=Dictionary&va=chemistry

To be a scientist or chemist… • What do you think of when you think of chemists?

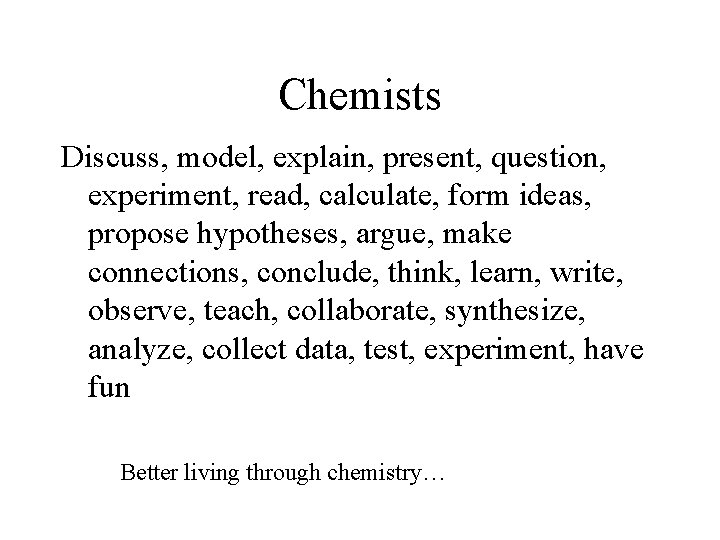
Chemists Discuss, model, explain, present, question, experiment, read, calculate, form ideas, propose hypotheses, argue, make connections, conclude, think, learn, write, observe, teach, collaborate, synthesize, analyze, collect data, test, experiment, have fun Better living through chemistry…

Chemistry…. • What do you expect from this course? • What do you want to be able to do?

Course Goals To Be Chemists: 1) Chemistry concepts 2) Analysis & Connections 3) Creativity 4) Problem Solving 5) Communication and team work

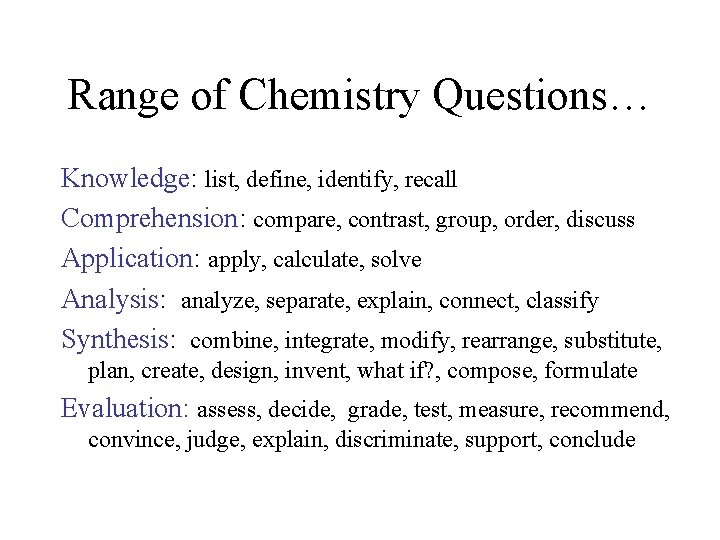
Range of Chemistry Questions… Knowledge: list, define, identify, recall Comprehension: compare, contrast, group, order, discuss Application: apply, calculate, solve Analysis: analyze, separate, explain, connect, classify Synthesis: combine, integrate, modify, rearrange, substitute, plan, create, design, invent, what if? , compose, formulate Evaluation: assess, decide, grade, test, measure, recommend, convince, judge, explain, discriminate, support, conclude

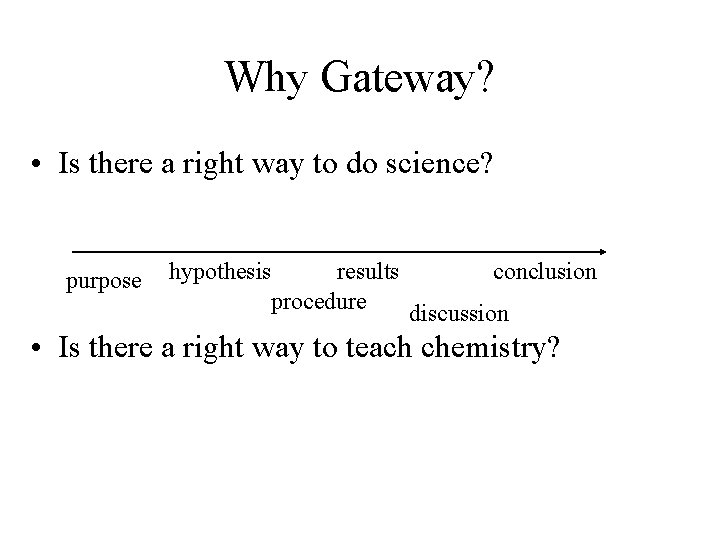
Why Gateway? • Is there a right way to do science? purpose hypothesis results conclusion procedure discussion • Is there a right way to teach chemistry?

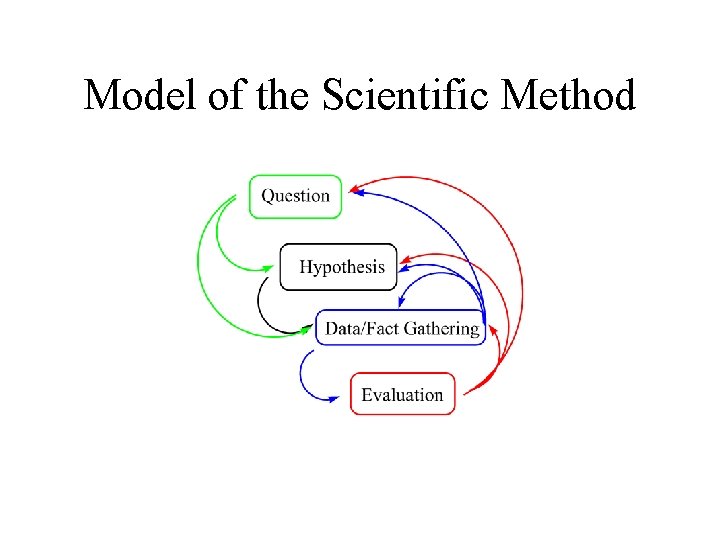
Model of the Scientific Method

Gateway Chemistry 125, 126, 130 Section 600

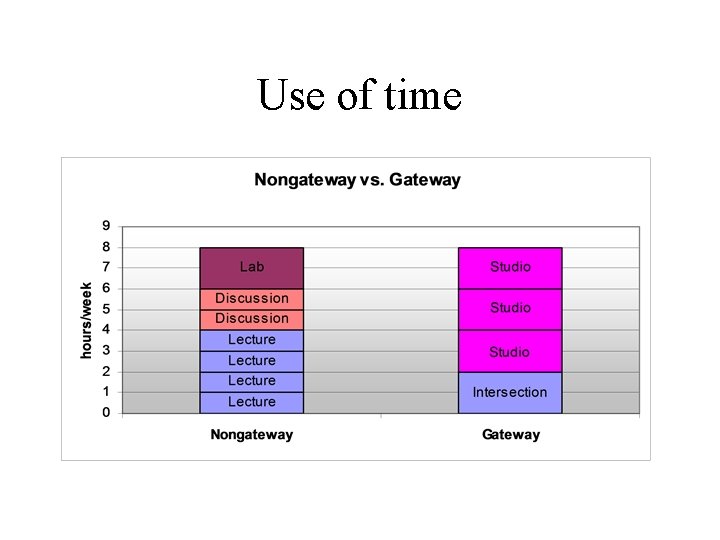
Use of time

Course components • Intersection: Concept questions, lecture, problem solving • Studio: discussion, activities, models, presentation, peer evaluation, models (learning and applying) problem solving, case studies, experimentation

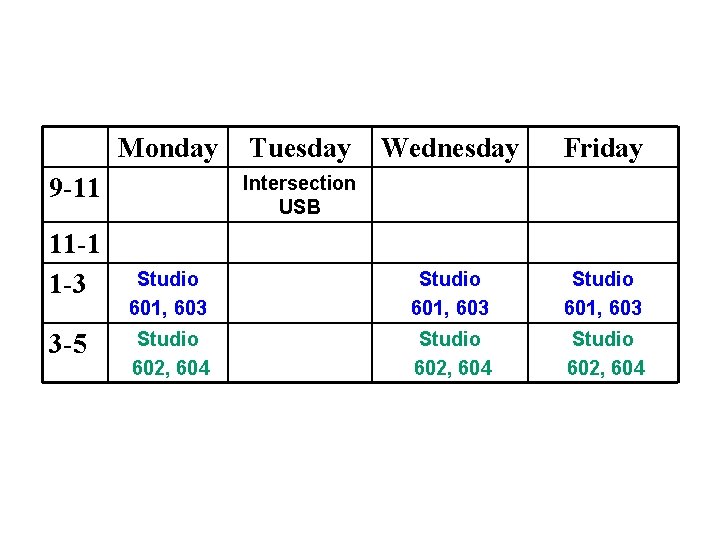
Monday Wednesday Friday Studio 601, 603 Studio 602, 604 Intersection USB 9 -11 11 -1 1 -3 3 -5 Tuesday

Integrated Lecture and Lab • Make connections • A scientist isn’t a scientist without doing things that scientists do • Chemists don’t separate problem solving or concepts from experimentation; they actually work in a studio like environment • Could I teach you how to drive a car without getting behind the wheel? Picture from: http: //images. google. com/imgres? imgurl=http: //www. autobytel. com/images/Autoshows/lashow/650/DSCN 0174. jpg&imgrefurl=http: //www. autobytel. com/content/research/index. cfm/action/show. Article/aid/139115&h=372&w=650&sz=25&tbnid=R 3 az. SFCBe. CQJ: &tbnh=77&tbn w=135&hl=en&start=16&prev=/images%3 Fq%3 Dcar%26 hl%3 Den%26 lr%3 D%26 sa%3 DN

Why teach like this? • Average attention span is 20 minutes • Cooperative learning has been shown to help students increase learning • People learn in a variety of ways • Prepare you for future challenges that will involve chemistry (be it classes or not) http: //main. uab. edu/show. asp? durki=1479 http: //www. doctorsecrets. com/secrets-in-medicine/medical-school. html

Other Gateway Stuff The website: http: //www. umich. edu/~chemstu Calendar: http: //www. umich. edu/~chemstu/calendar _monthly_sept. htm

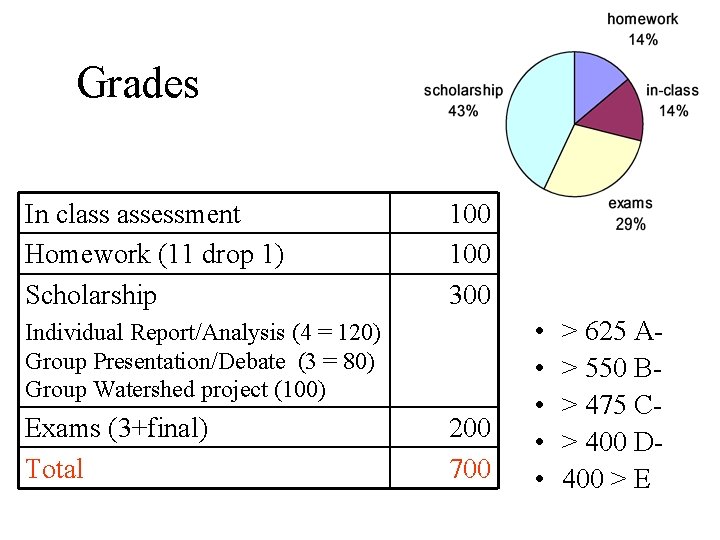
Grades In class assessment Homework (11 drop 1) Scholarship 100 300 Individual Report/Analysis (4 = 120) Group Presentation/Debate (3 = 80) Group Watershed project (100) Exams (3+final) Total 200 700 • • • > 625 A> 550 B> 475 C> 400 D 400 > E

• Readings • Homework: – 11 assignments, drop lowest grade. – Due at the beginning of studio on Wednesday. No late homework (after 1: 10 or 3: 10) will be accepted. – Grading: 4 points for completing all of the assignment, 3 points each for two random problems that will be graded. • Exams – 6 -8 pm on Tuesdays – Points for Exams: 40, 45, 65

In-class points – Earn up to 100 in-class points – Coursepack or to hand in a short writing assignment. – Points may come for individual or group work. 0 -for a physical absence; OR endangered self or others through safety violation 1 -participation has room much for improvement; work partially complete; OR does not clean up area before leaving 2 -sometimes mentally unengaged; runs long or rushes through work 3 -good day's work; helps others, particularly group members; engaged throughout class time; working to show learning and improvement 4 -excellent work above and beyond what was expected; thought creatively and made great connections

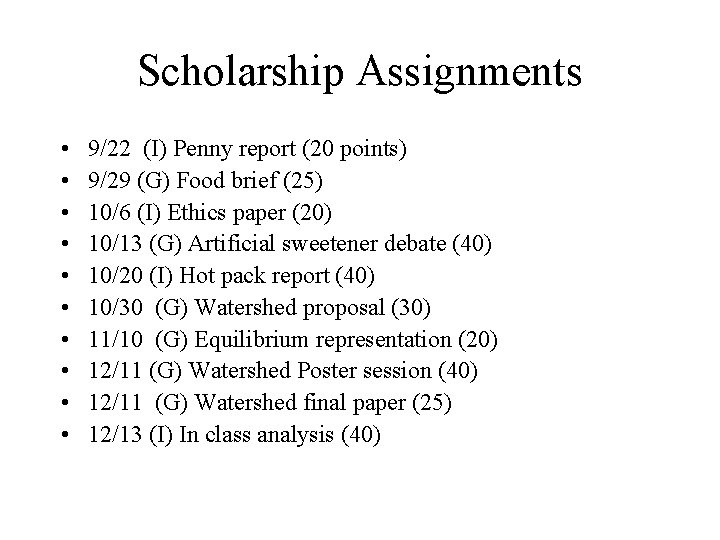
Scholarship Assignments • • • 9/22 (I) Penny report (20 points) 9/29 (G) Food brief (25) 10/6 (I) Ethics paper (20) 10/13 (G) Artificial sweetener debate (40) 10/20 (I) Hot pack report (40) 10/30 (G) Watershed proposal (30) 11/10 (G) Equilibrium representation (20) 12/11 (G) Watershed Poster session (40) 12/11 (G) Watershed final paper (25) 12/13 (I) In class analysis (40)

Miscellaneous • 5 hour course= 10 hours a week outside of class on work! • Attendance • Academic integrity • Safety

As we get started Need for gateway – Coursepack Dollar Bill Copying M-TH. 9 am 8 pm, Friday 9 am-5 pm, and Noon - 5 pm on Saturday and Sunday – Text: Moore, Stanitski, and Jurs Chemistry 2 nd Edition – Non-programmable calculator


Question 1 Assume a beaker of pure water has been boiling for 30 minutes. What is in the bubbles in the boiling water? a. Air. b. Oxygen gas and hydrogen gas. c. Oxygen. d. Water vapor. e. Heat.

Question 2 What is the mass of the solution when 1 pound of salt is dissolved in 20 pounds of water? a. 19 Pounds. b. 20 Pounds. c. Between 20 and 21 pounds. d. 21 pounds. e. More than 21 pounds.

Question 3 1) As a candle burns, it gives off light and heat. When a glass rod is held in the yellow part of the flame, a black film forms on the rod. a) What is the source of the black film on the rod? b) Is there a chemical change or a physical change in the candle as it burns? c) Give an example of a chemical change: d) Give an example of a physical change

Question 4 • There are two identical steel beams. One is placed on each side of a balance. A flame is used to heat one of the steel beams. Does the balance move? If so, how and why?

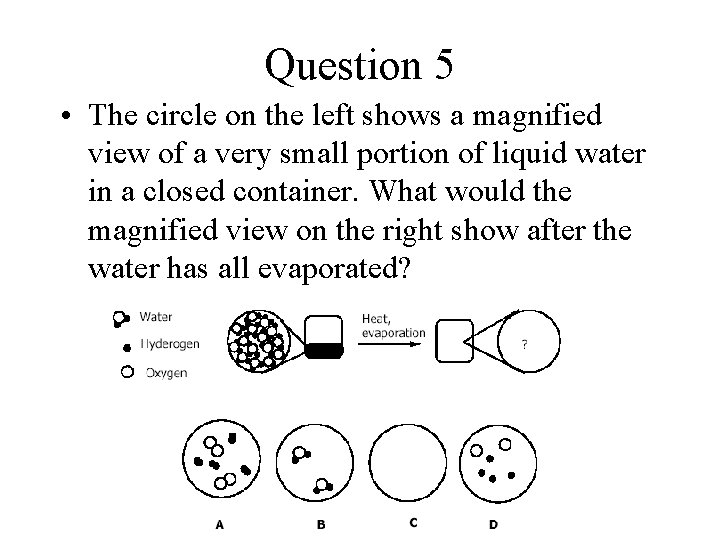
Question 5 • The circle on the left shows a magnified view of a very small portion of liquid water in a closed container. What would the magnified view on the right show after the water has all evaporated?


Define the following: • • • Fact Law Theory Hypothesis Model

According to the National Academy of Science, a Hypothesis is: A testable statement about the natural world that can be used to build more complex inferences and explanations These definitions (and an excellent explanation of the scientific method) can be found at: http: //www. nap. edu/readingroom/books/evolution 98/

According to the National Academy of Science, a Fact is: In science, an observation that has been repeatedly confirmed.

According to the National Academy of Science, a Law is: A descriptive generalization about how some aspect of the natural world behaves under stated circumstances How are a fact and a law related? Can you think of any scientific laws?

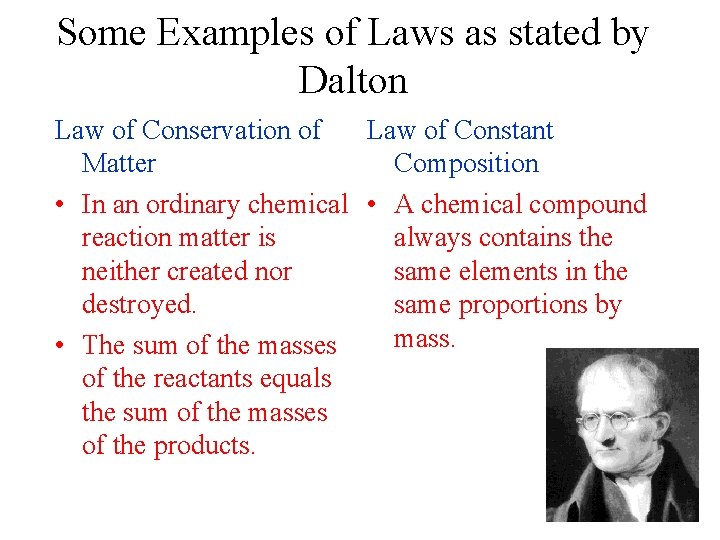
Some Examples of Laws as stated by Dalton Law of Conservation of Law of Constant Matter Composition • In an ordinary chemical • A chemical compound reaction matter is always contains the neither created nor same elements in the destroyed. same proportions by mass. • The sum of the masses of the reactants equals the sum of the masses of the products.

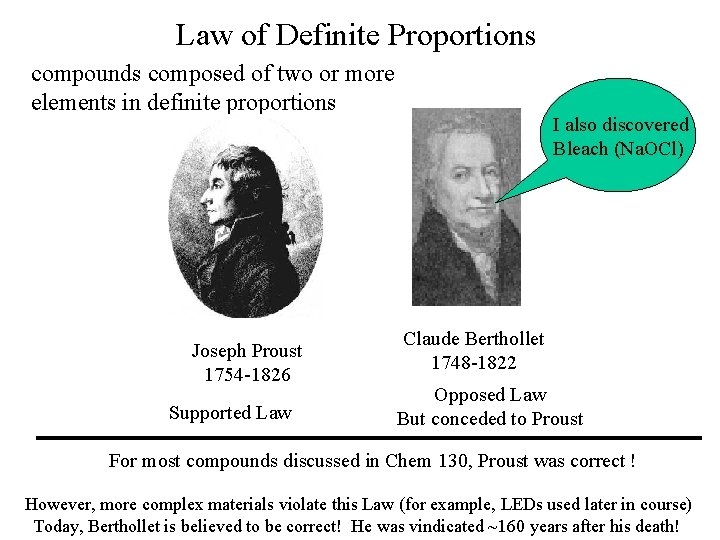
“Laws” Can Be Overturned ! Which Law from previous page is now known to be incorrect ? Law of Conservation of Matter Law of Constant Composition The “Law of Constant Composition” also has an older name given by Joseph Proust. The Law of Definite Proportions Once a Law has become accepted, it is very difficult to get it convince the scientific community to discard it. Hence, this one still appears in your textbook despite its limitations. This law is only true for simple, small molecules.

Law of Definite Proportions compounds composed of two or more elements in definite proportions Joseph Proust 1754 -1826 Supported Law I also discovered Bleach (Na. OCl) Claude Berthollet 1748 -1822 Opposed Law But conceded to Proust For most compounds discussed in Chem 130, Proust was correct ! However, more complex materials violate this Law (for example, LEDs used later in course) Today, Berthollet is believed to be correct! He was vindicated ~160 years after his death!

According to the National Academy of Science, a Theory is In science, a well-substantiated explanation of some aspect of the natural world that can incorporate facts, laws, inferences, and tested hypotheses. Theories must be falsifiable.

According to the National Academy of Science, a Model is A description or analogy used to help visualize something (as an atom) that cannot be directly observed

Theory vs. Model • Theory based on facts, evidence • Model is the picture, an analogy, a way of describing a theory


Qualitative vs. Quantitative • Fehling’s Reagent http: //jchemed. chem. wisc. edu/JCESoft/CCA/ CCA 0/MOVIES/FEHLTEST. html

Take home lessons • “Give a man a fish and you feed him for a day. Teach a man to fish and you feed him for a lifetime. ” – Chinese proverb • "Learning is not attained by chance, it must be sought for with ardor and attended to with diligence. " --- Abigail Adams • "Imagination is more important than knowledge. " -Albert Einstein

Actions You Need to Take • Read the course syllabus • Get a coursepack! • Begin Homework 1 – Due Monday, 9/11