Changes in states of matter pt 1 Change







- Slides: 17

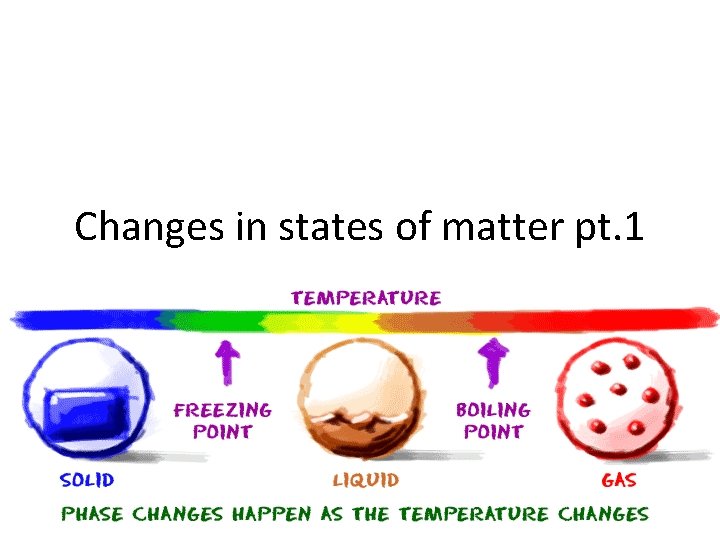
Changes in states of matter pt. 1

Change of state, AKA phase change • A change from one state of matter to another is a result of two things –Changes in the motion of the particles –Strength of the forces between the particles

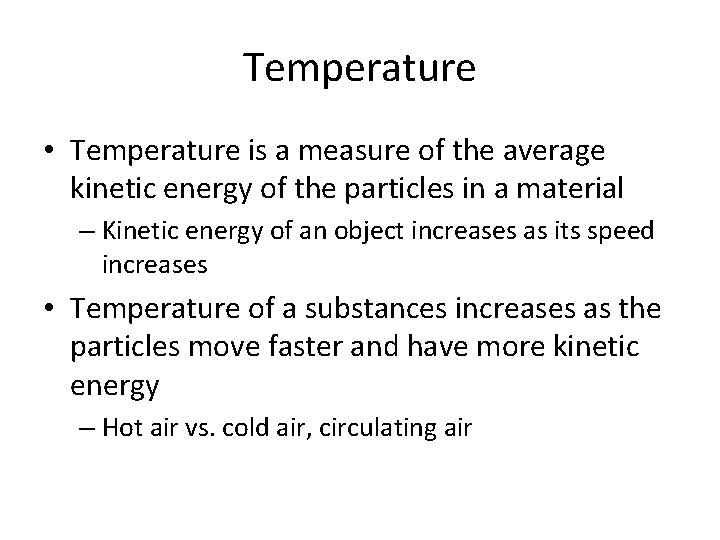
Temperature • Temperature is a measure of the average kinetic energy of the particles in a material – Kinetic energy of an object increases as its speed increases • Temperature of a substances increases as the particles move faster and have more kinetic energy – Hot air vs. cold air, circulating air


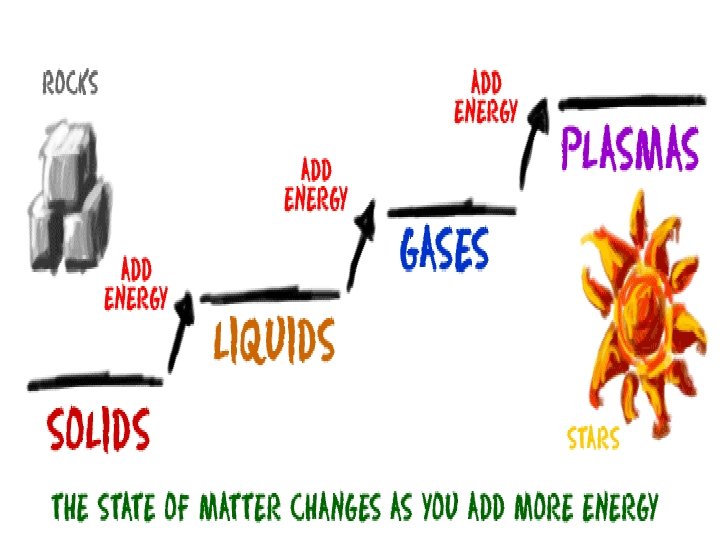
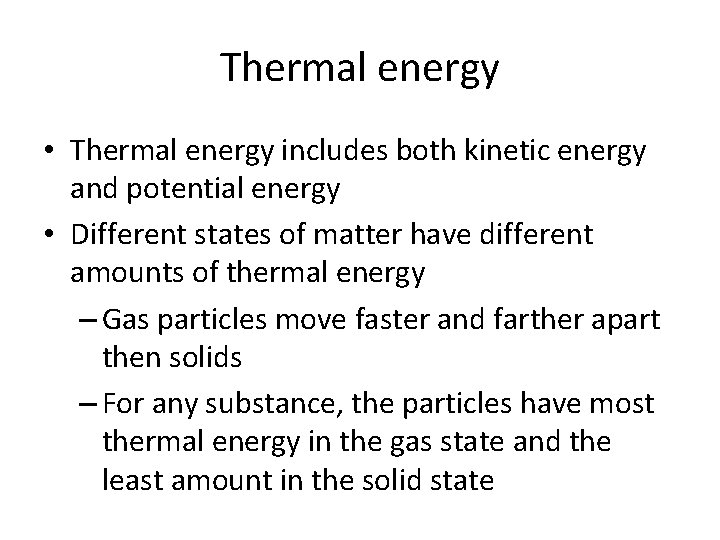
Thermal energy • Thermal energy includes both kinetic energy and potential energy • Different states of matter have different amounts of thermal energy – Gas particles move faster and farther apart then solids – For any substance, the particles have most thermal energy in the gas state and the least amount in the solid state

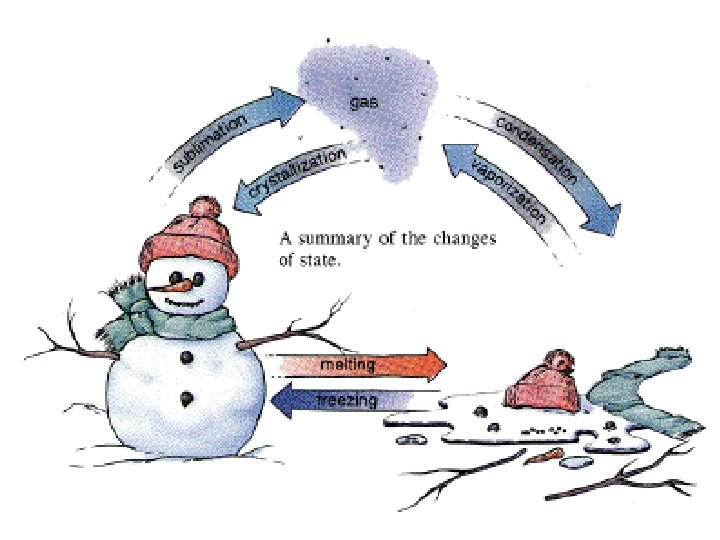
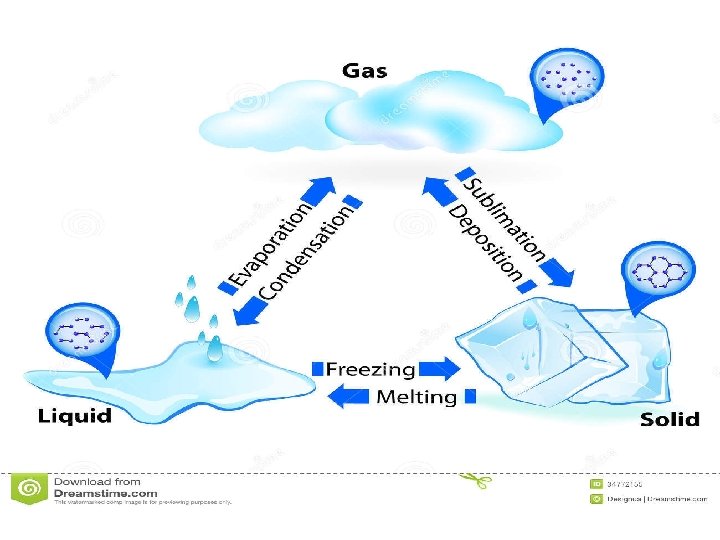
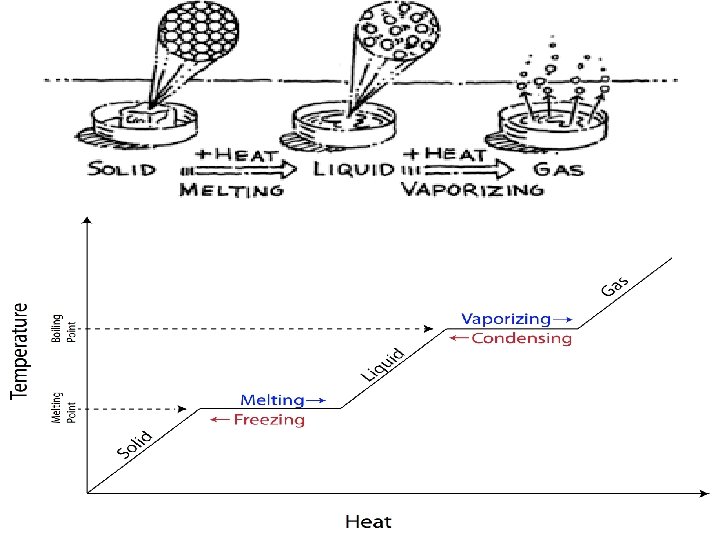
Adding and removing thermal energy • Adding thermal energy to a substance can cause either an increase in temperature or a change in state • A change in state is when matter changes from one state to another – Solid to liquid, liquid to solid – Liquid to gas, gas to liquid – Gas to solid, solid to gas



Changes of state pt. 2 review of states of matter

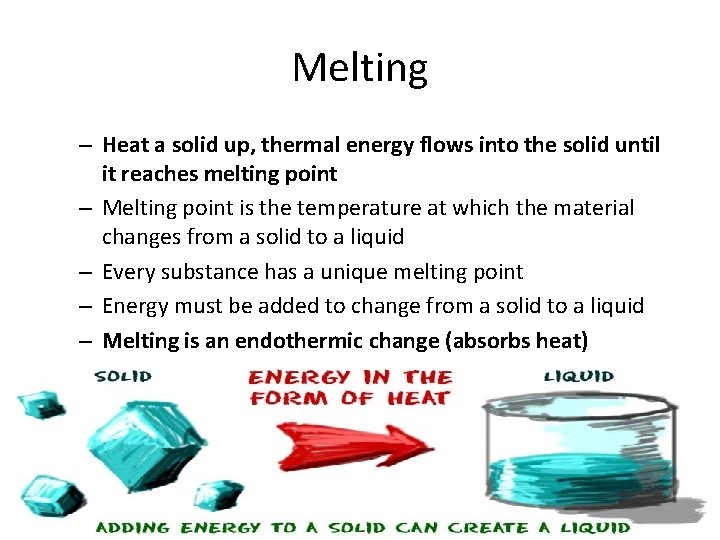
Melting – Heat a solid up, thermal energy flows into the solid until it reaches melting point – Melting point is the temperature at which the material changes from a solid to a liquid – Every substance has a unique melting point – Energy must be added to change from a solid to a liquid – Melting is an endothermic change (absorbs heat)

Freezing • Liquid changes into a solid • Material cools, thermal energy flows out of the material • Freezing point is the temperature at which the liquid changes into a solid • Opposite of melting, exothermic reaction (releases heat)

Vaporization and boiling • Liquid to a gas (vaporization) • For water, 100 degrees Celsius is boiling point, when it starts to evaporate • When vaporization occurs, attractive forces between particles become too weak to keep particles close to each. They spread out and move independently • Evaporations occurs at the surface of a liquid

Condensation • Gas to a liquid • Large number of atoms /molecules clump together • On a hot day you might see drops of water on the outside of a glass of ice cold water • Dew in the morning is another example • Reverse of vaporization, exothermic reaction (releases energy)

Sublimation and deposition • Solid to a gas is sublimation, without going to liquid state • Dry ice is an example • Thermal energy must be added to a solid • Gas to solid is deposition • Frost that forms on a leaf is an example


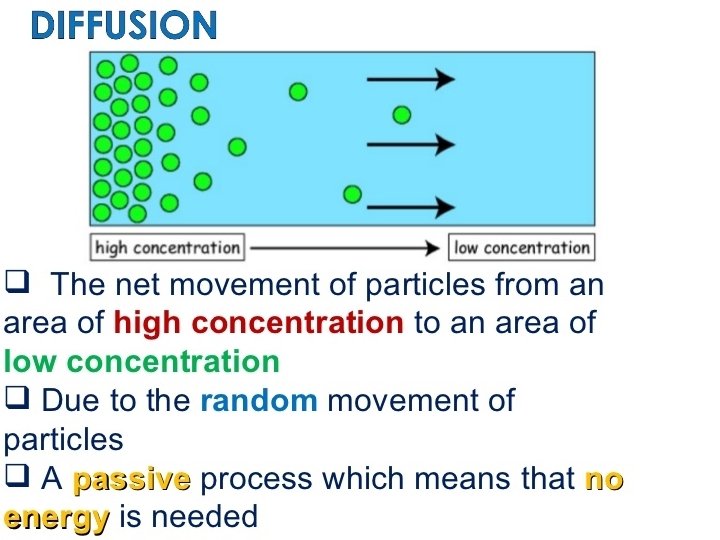
Diffusion • Diffusion is the net movement of particles from an area of high concentration to an area low concentration • Can occur with the states of matter, either by themselves or when mixing • Diffusion video
