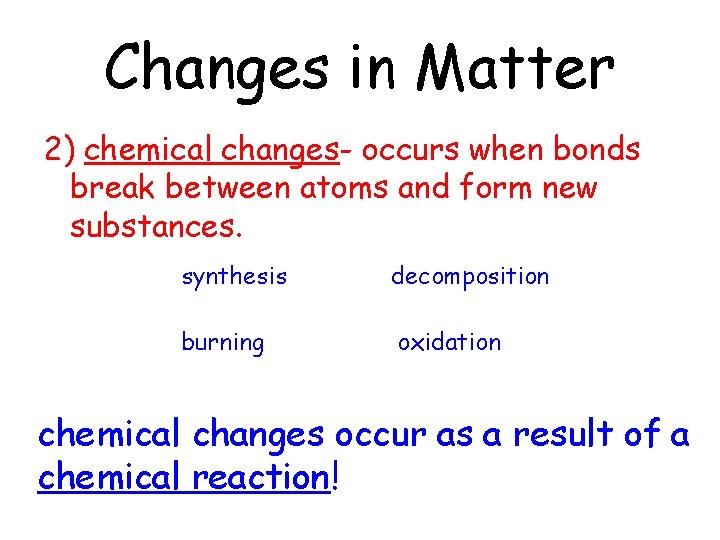
Changes in Matter 2 chemical changes occurs when


- Slides: 15

Changes in Matter 2) chemical changes- occurs when bonds break between atoms and form new substances. synthesis decomposition burning oxidation chemical changes occur as a result of a chemical reaction!

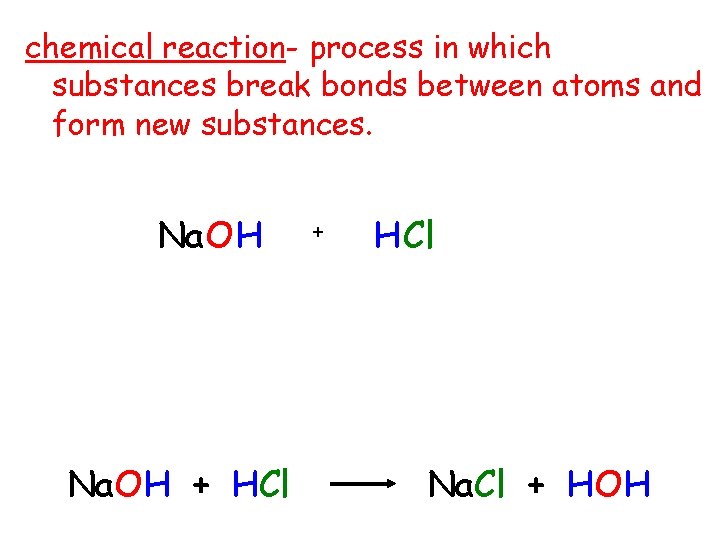
chemical reaction- process in which substances break bonds between atoms and form new substances. Na. OH + HCl + H Cl Na. Cl + HOH

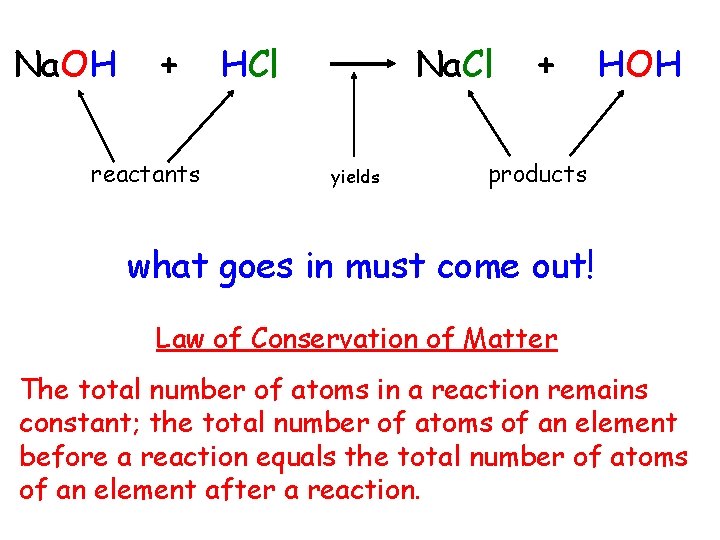
Na. OH + reactants HCl Na. Cl yields + HOH products what goes in must come out! Law of Conservation of Matter The total number of atoms in a reaction remains constant; the total number of atoms of an element before a reaction equals the total number of atoms of an element after a reaction.

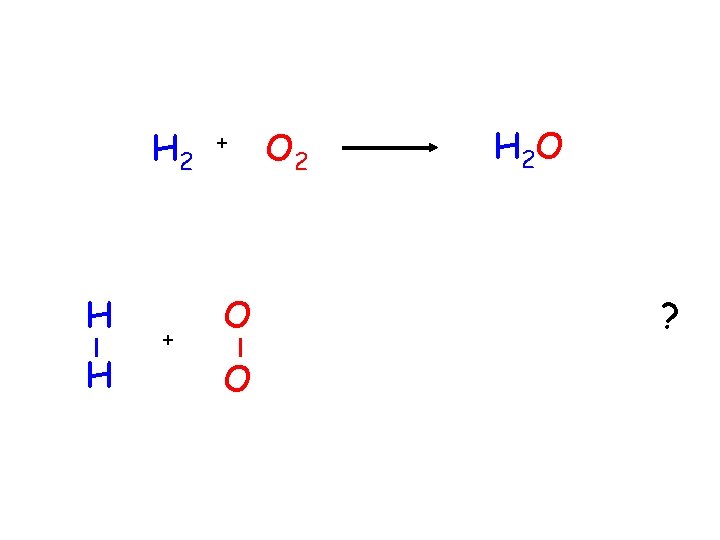
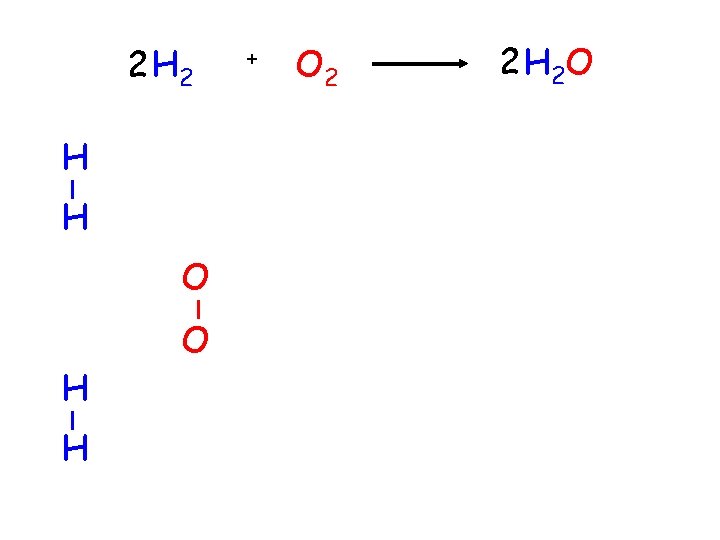
H 2 H H + + O O O 2 H 2 O ?

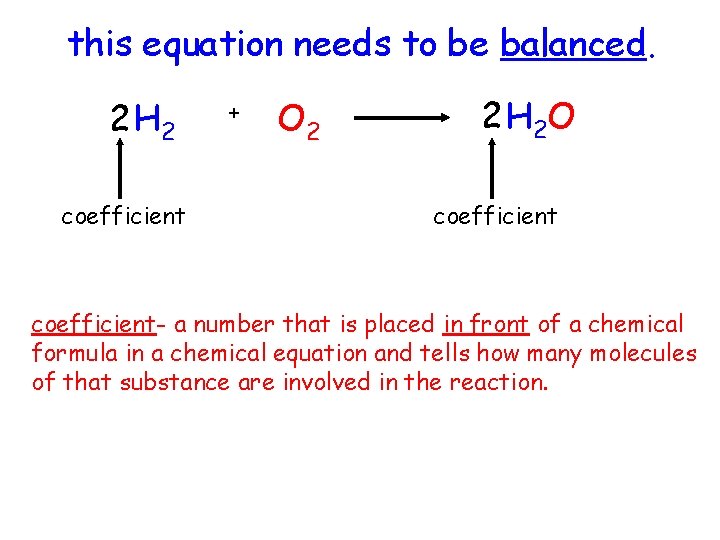
this equation needs to be balanced. 2 H 2 coefficient + O 2 2 H 2 O coefficient- a number that is placed in front of a chemical formula in a chemical equation and tells how many molecules of that substance are involved in the reaction.

2 H H O + O 2 2 H 2 O

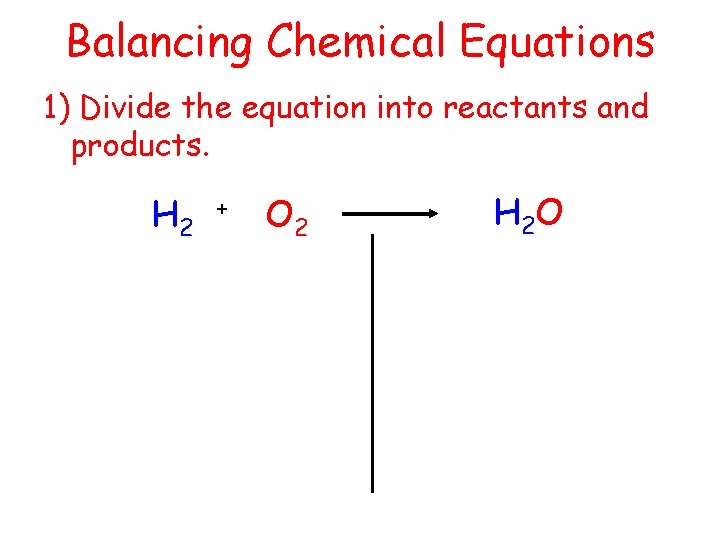
Balancing Chemical Equations 1) Divide the equation into reactants and products. H 2 + O 2 H 2 O

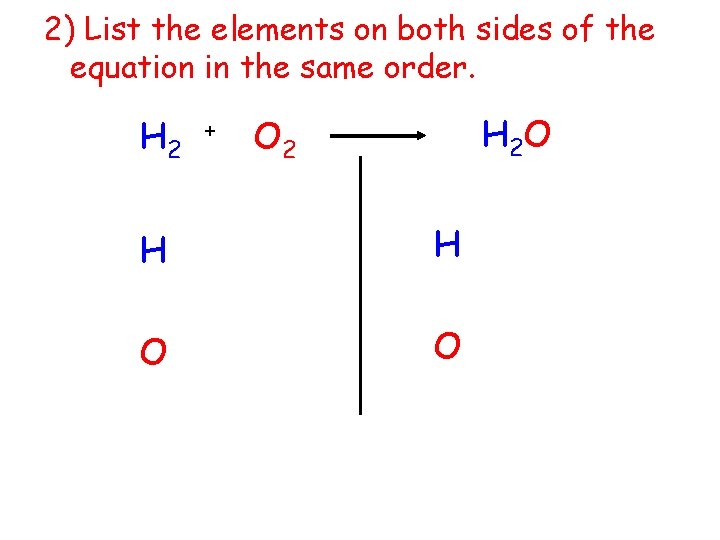
2) List the elements on both sides of the equation in the same order. H 2 + H 2 O O 2 H H O O

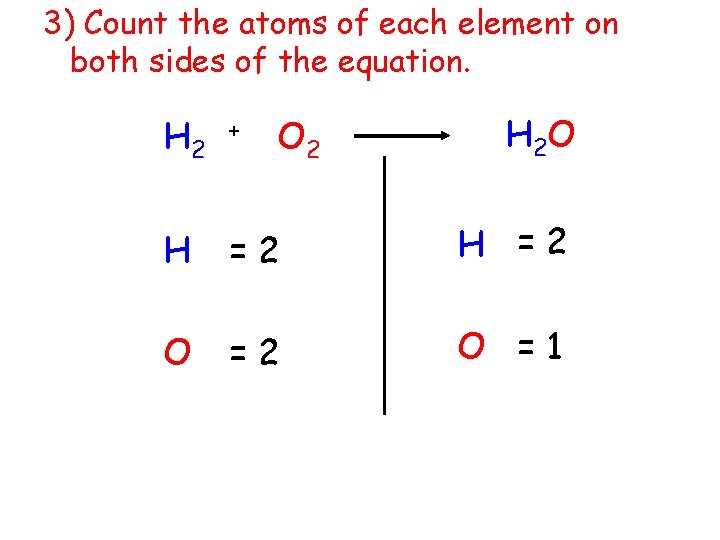
3) Count the atoms of each element on both sides of the equation. O 2 H 2 O H 2 + H =2 O =1

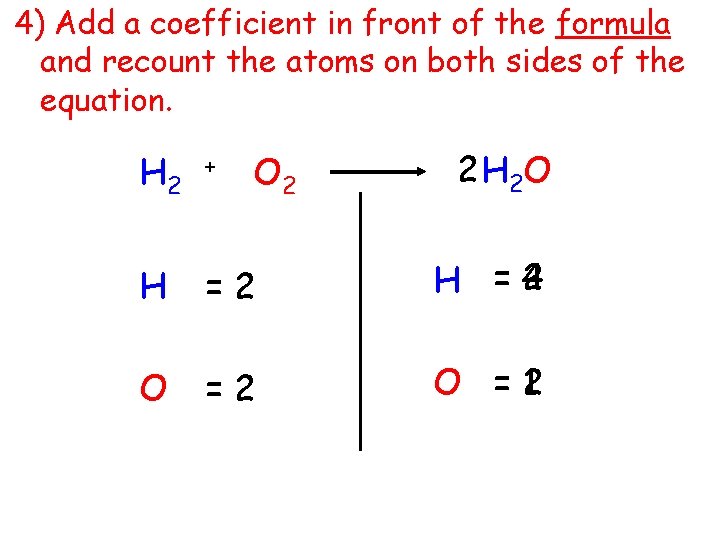
4) Add a coefficient in front of the formula and recount the atoms on both sides of the equation. O 2 2 H 2 O H 2 + H =2 2 H =4 O =2 1

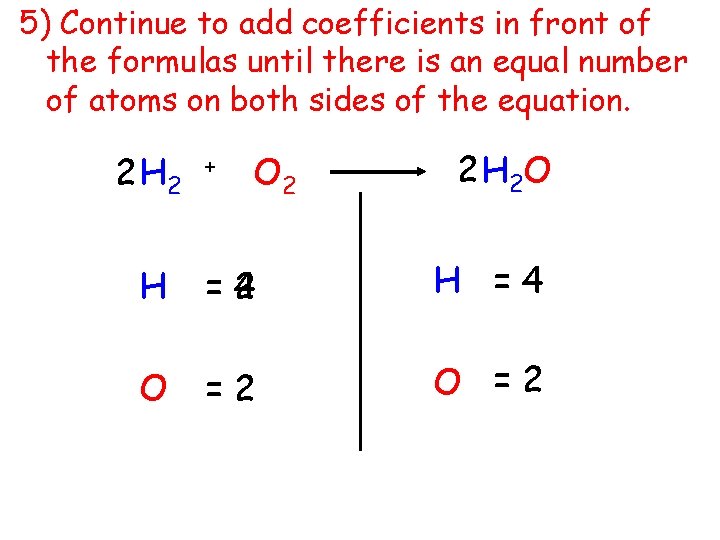
5) Continue to add coefficients in front of the formulas until there is an equal number of atoms on both sides of the equation. 2 H 2 + O 2 2 H 2 O H =4 2 H =4 O =2

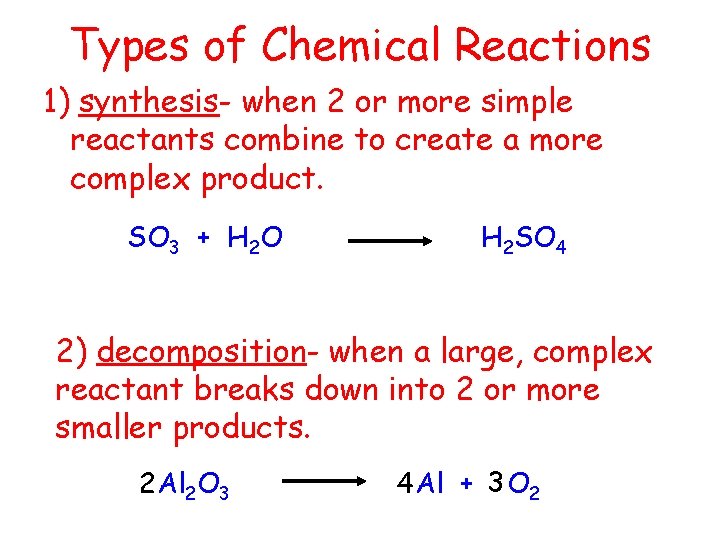
Types of Chemical Reactions 1) synthesis- when 2 or more simple reactants combine to create a more complex product. SO 3 + H 2 O H 2 SO 4 2) decomposition- when a large, complex reactant breaks down into 2 or more smaller products. 2 Al 2 O 3 4 Al + 3 O 2

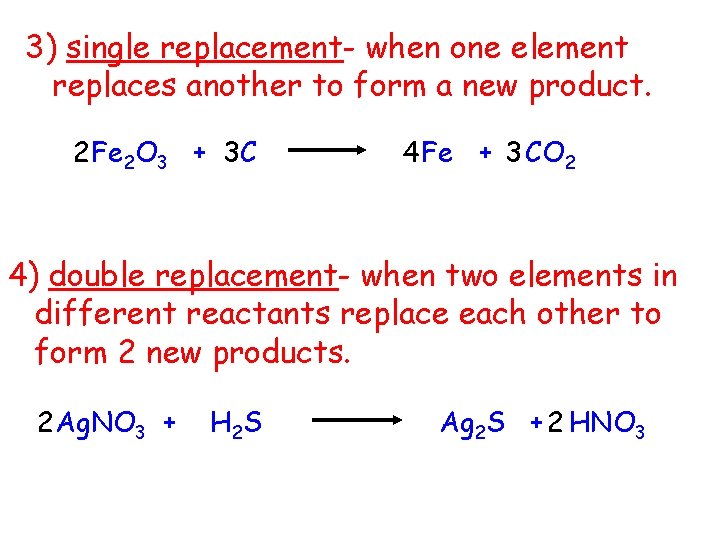
3) single replacement- when one element replaces another to form a new product. 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2 4) double replacement- when two elements in different reactants replace each other to form 2 new products. 2 Ag. NO 3 + H 2 S Ag 2 S + 2 HNO 3

Reactions & Energy endothermic reactions- chemical reactions that absorb energy; feels cold. exothermic reactions- chemical reactions that release energy (light &/or heat); feels warm.
