Changes in Matter 2 3 n PHYSICAL CHANGE


- Slides: 10

Changes in Matter 2. 3 n PHYSICAL CHANGE -a substance that undergoes a physical change is still the same substance after the change -changes of state -solid, liquid, gas -changes in shape or form -dissolving, bending, crushing, breaking, chopping, etc.

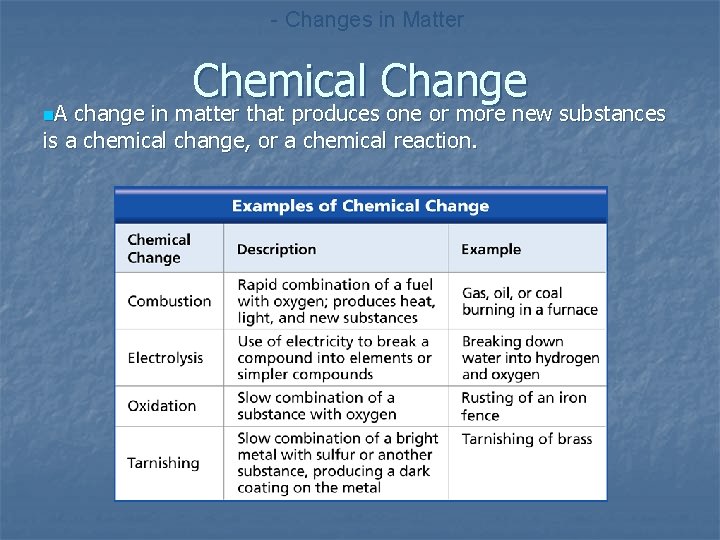
n n CHEMICAL CHANGE -produces one or more new substances -combustion, electrolysis, oxidation, tarnishing MATTER & THERMAL ENERGY -every physical or chemical change includes a change in energy -endothermic changes -exothermic changes

- Changes in Matter n. A Chemical Change change in matter that produces one or more new substances is a chemical change, or a chemical reaction.

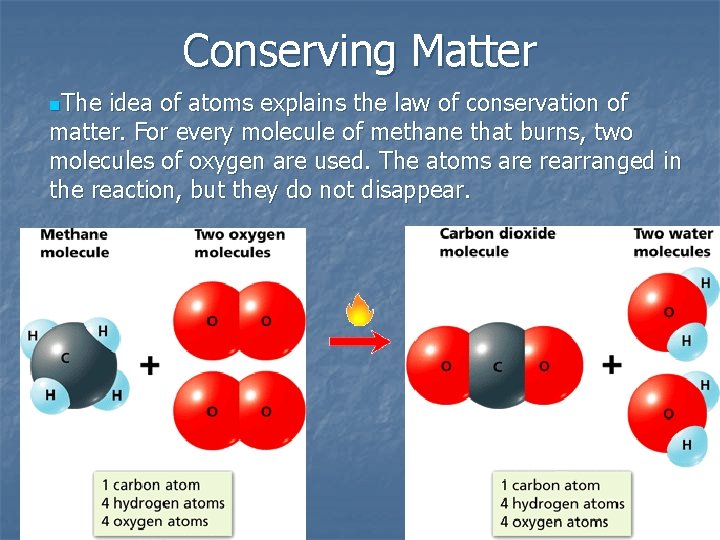
Conserving Matter n. The idea of atoms explains the law of conservation of matter. For every molecule of methane that burns, two molecules of oxygen are used. The atoms are rearranged in the reaction, but they do not disappear.

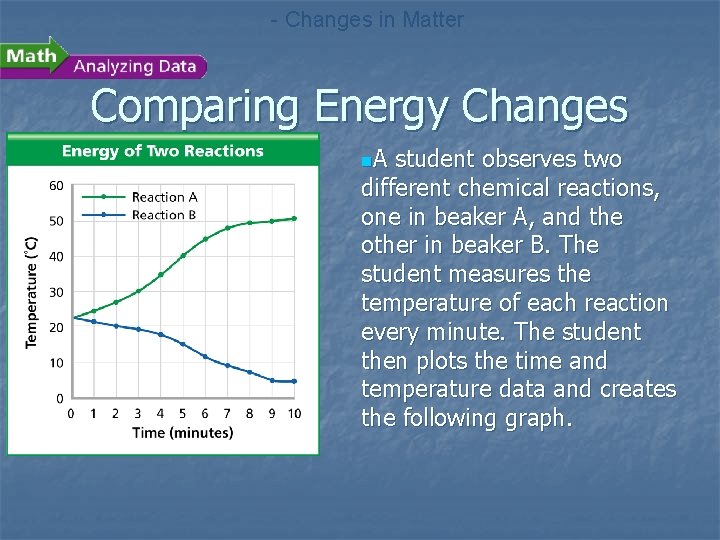
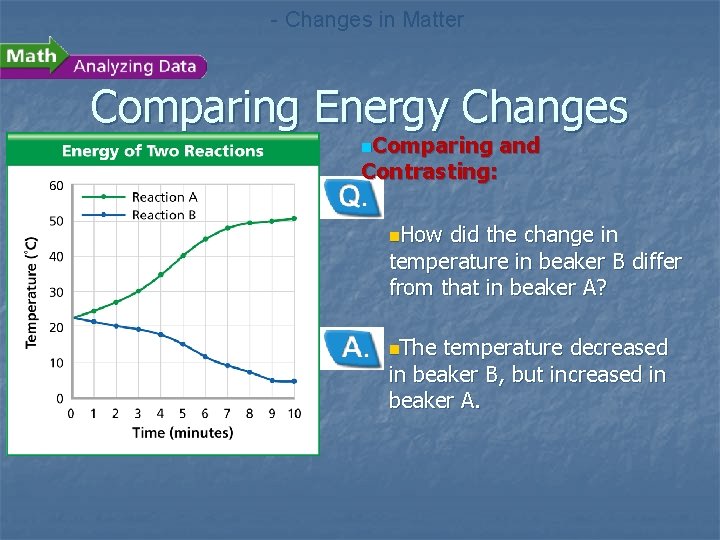
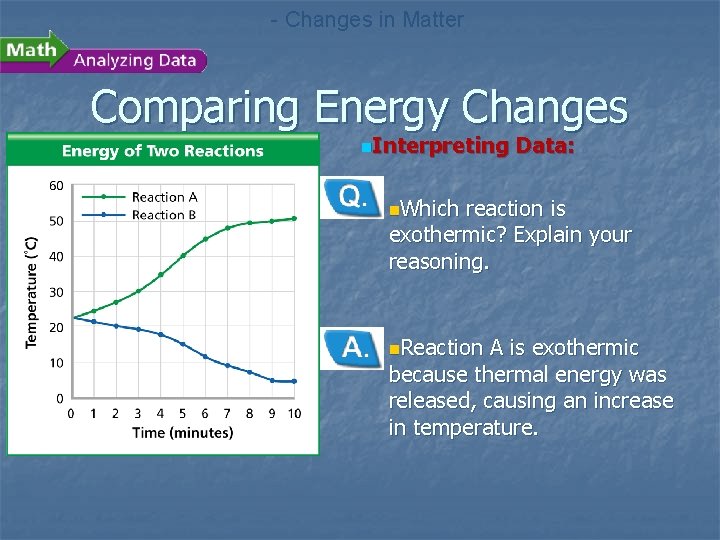
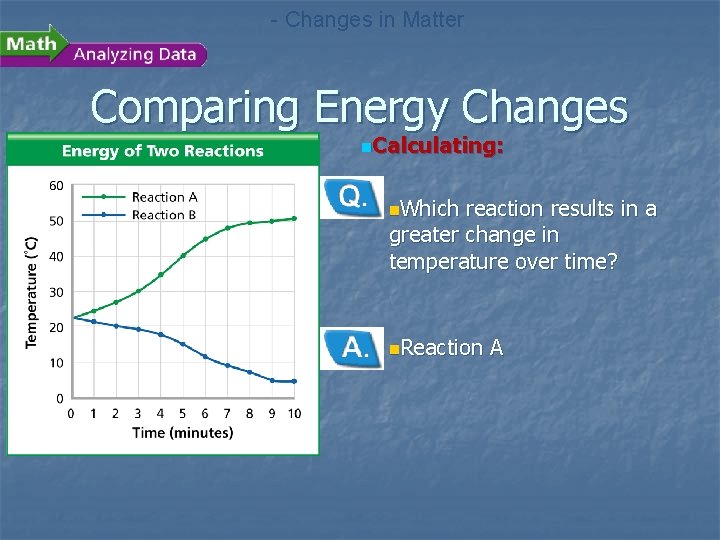
- Changes in Matter Comparing Energy Changes n. A student observes two different chemical reactions, one in beaker A, and the other in beaker B. The student measures the temperature of each reaction every minute. The student then plots the time and temperature data and creates the following graph.

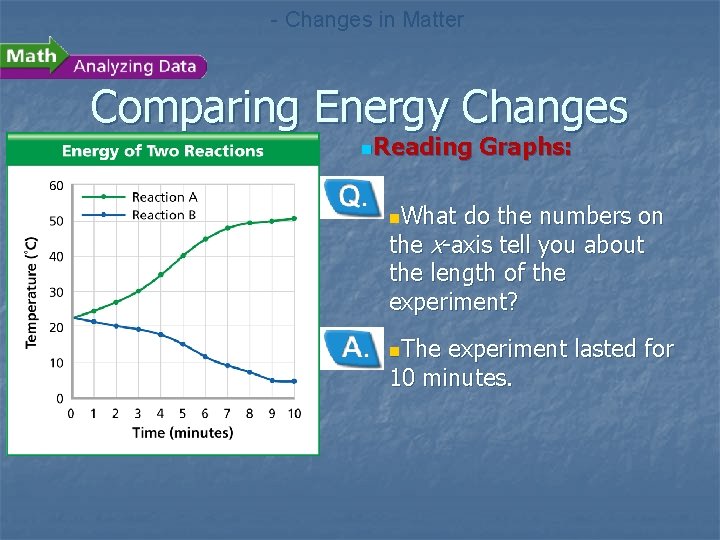
- Changes in Matter Comparing Energy Changes n. Reading Graphs: n. What do the numbers on the x-axis tell you about the length of the experiment? n. The experiment lasted for 10 minutes.

- Changes in Matter Comparing Energy Changes n. Comparing and Contrasting: n. How did the change in temperature in beaker B differ from that in beaker A? n. The temperature decreased in beaker B, but increased in beaker A.

- Changes in Matter Comparing Energy Changes n. Interpreting Data: n. Which reaction is exothermic? Explain your reasoning. n. Reaction A is exothermic because thermal energy was released, causing an increase in temperature.

- Changes in Matter Comparing Energy Changes n. Calculating: n. Which reaction results in a greater change in temperature over time? n. Reaction A

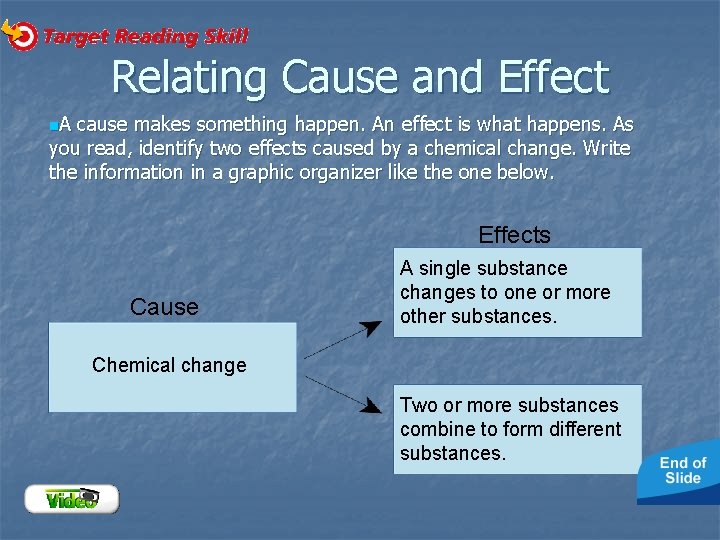
Relating Cause and Effect n. A cause makes something happen. An effect is what happens. As you read, identify two effects caused by a chemical change. Write the information in a graphic organizer like the one below. Effects Cause A single substance changes to one or more other substances. Chemical change Two or more substances combine to form different substances.