CHANGES IN FDA TECHNICAL CONFORMANCE GUIDE V 4











- Slides: 25

CHANGES IN FDA TECHNICAL CONFORMANCE GUIDE V 4. 2/4. 2. 1 06. März 2019, German User Group Meeting, Marion Friebel CONFIDENTIAL © 2019 PAREXEL INTERNATIONAL CORP.

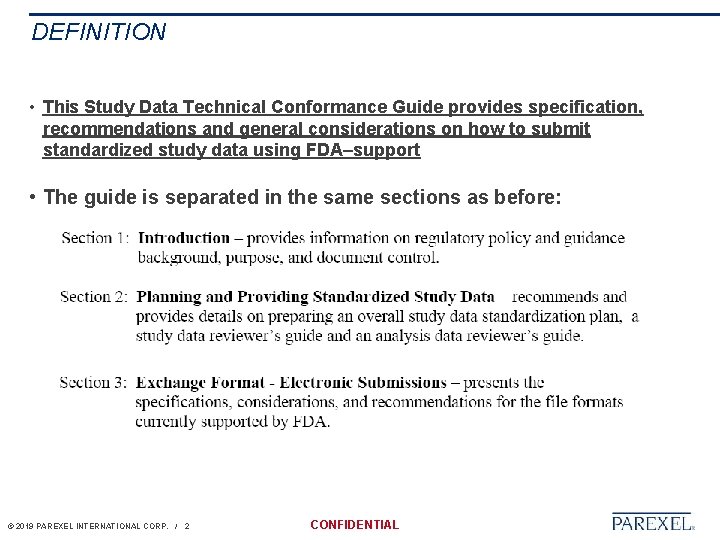
DEFINITION • This Study Data Technical Conformance Guide provides specification, recommendations and general considerations on how to submit standardized study data using FDA–support • The guide is separated in the same sections as before: © 2019 PAREXEL INTERNATIONAL CORP. / 2 CONFIDENTIAL

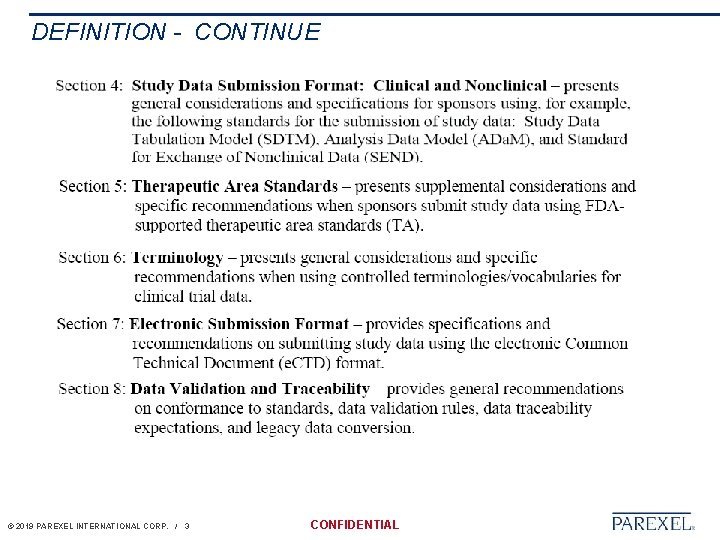
DEFINITION - CONTINUE © 2019 PAREXEL INTERNATIONAL CORP. / 3 CONFIDENTIAL

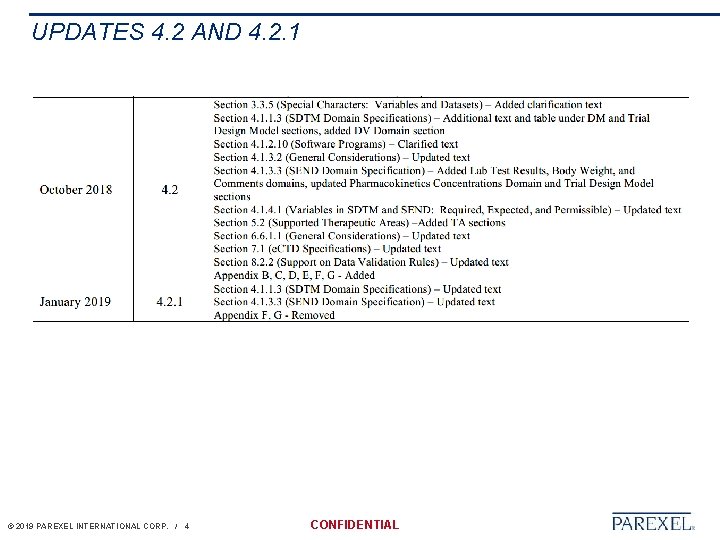
UPDATES 4. 2 AND 4. 2. 1 © 2019 PAREXEL INTERNATIONAL CORP. / 4 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 3. 3. 5 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 5 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 4. 1. 1. 3 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 6 CONFIDENTIAL

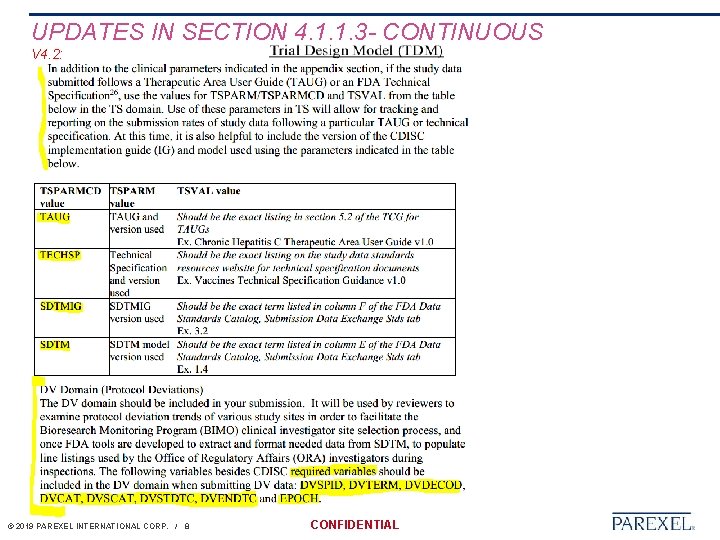
UPDATES IN SECTION 4. 1. 1. 3 - CONTINUOUS V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 7 CONFIDENTIAL

UPDATES IN SECTION 4. 1. 1. 3 - CONTINUOUS V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 8 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 4. 1. 1. 3 V 4. 1: v 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 9 CONFIDENTIAL

UPDATES IN SECTION 4. 1. 2. 10 - CONTINUOUS © 2019 PAREXEL INTERNATIONAL CORP. / 10 CONFIDENTIAL

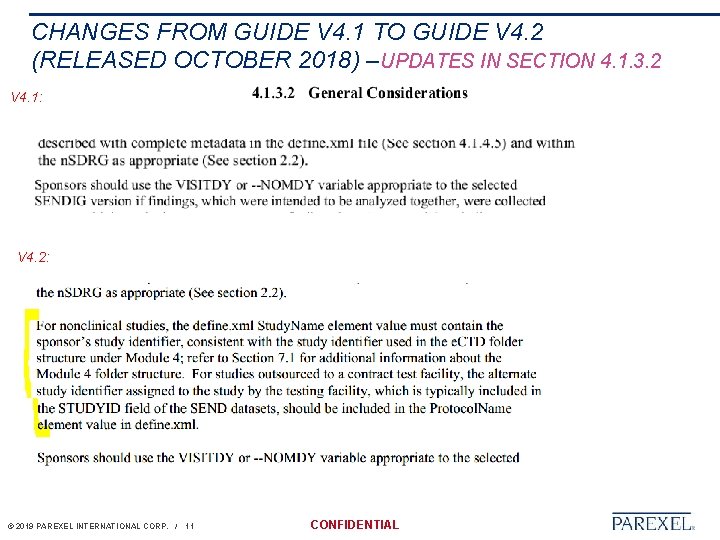
CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 4. 1. 3. 2 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 11 CONFIDENTIAL

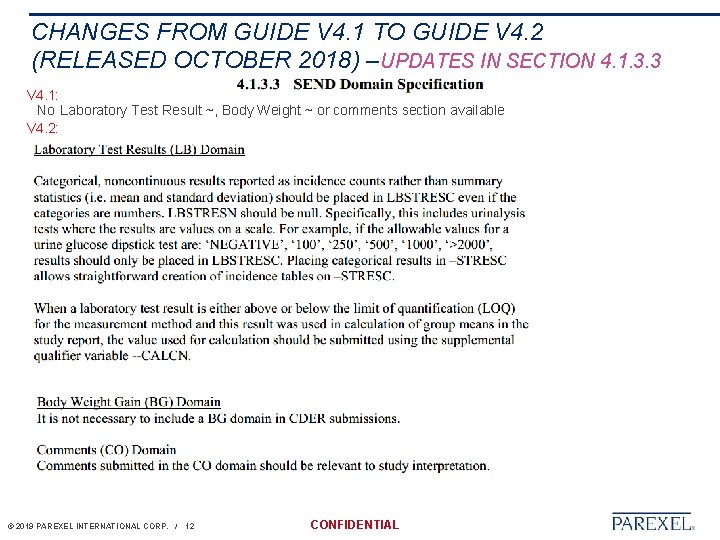
CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 4. 1. 3. 3 V 4. 1: No Laboratory Test Result ~, Body Weight ~ or comments section available V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 12 CONFIDENTIAL

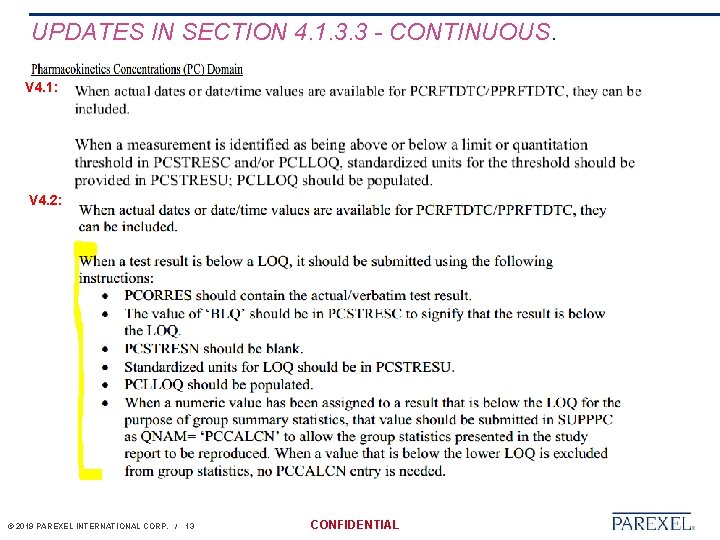
UPDATES IN SECTION 4. 1. 3. 3 - CONTINUOUS. V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 13 CONFIDENTIAL

UPDATES IN SECTION 4. 1. 3. 3 - CONTINUOUS. V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 14 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 4. 1 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 15 CONFIDENTIAL

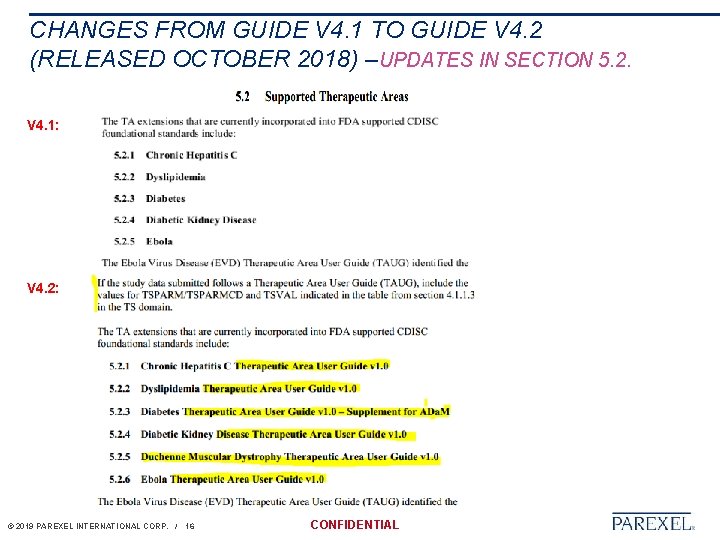
CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 5. 2. V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 16 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 6. 6. 1. 1 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 17 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 7. 1 V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 18 CONFIDENTIAL

CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2. 1 (RELEASED JANUARY 2019) –UPDATES IN SECTION 7. 3 V 4. 1: not available V 4. 2. 1: not available © 2019 PAREXEL INTERNATIONAL CORP. / 19 CONFIDENTIAL

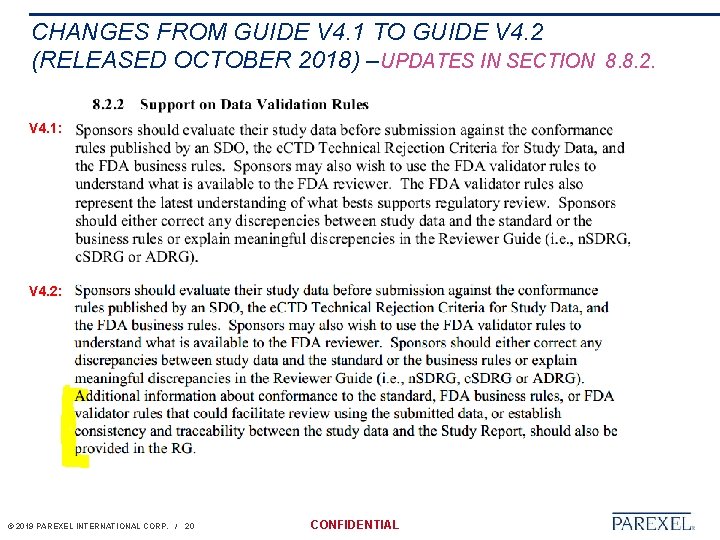
CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) –UPDATES IN SECTION 8. 8. 2. V 4. 1: V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 20 CONFIDENTIAL

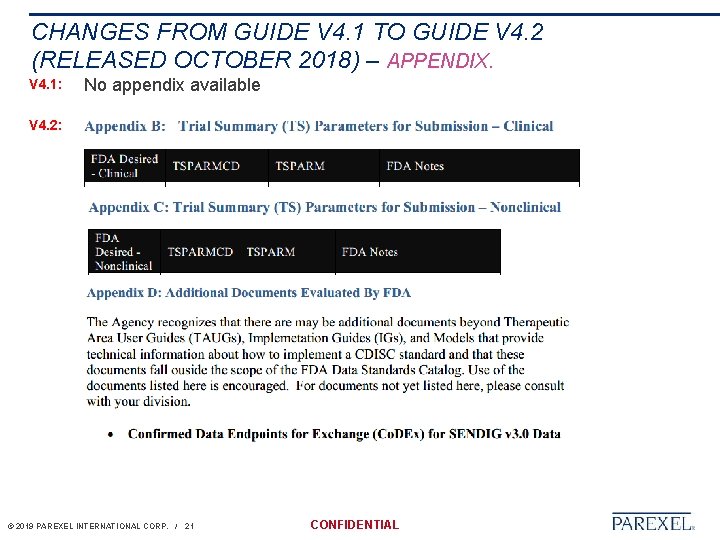
CHANGES FROM GUIDE V 4. 1 TO GUIDE V 4. 2 (RELEASED OCTOBER 2018) – APPENDIX. V 4. 1: No appendix available V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 21 CONFIDENTIAL

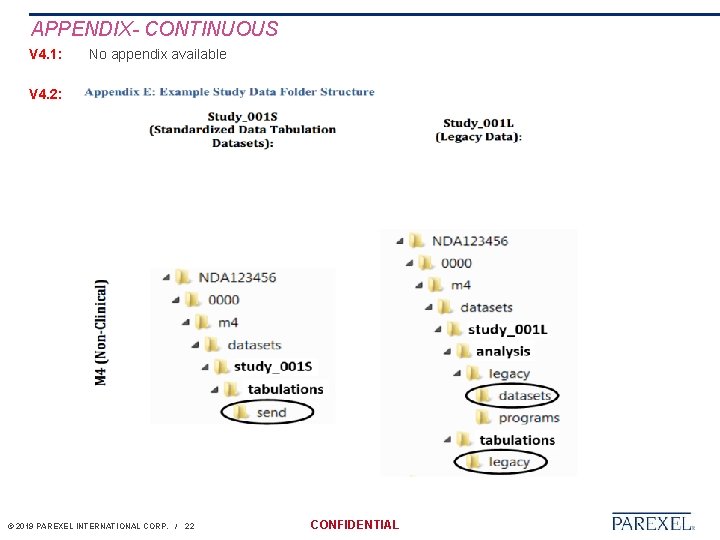
APPENDIX- CONTINUOUS V 4. 1: No appendix available V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 22 CONFIDENTIAL

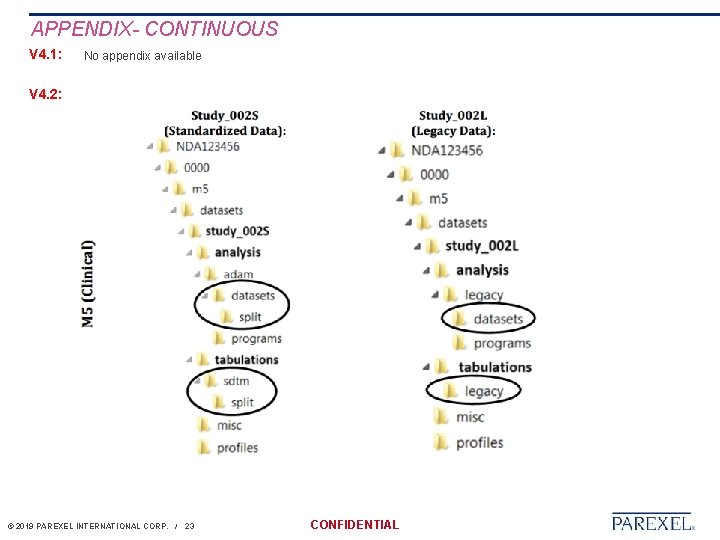
APPENDIX- CONTINUOUS V 4. 1: No appendix available V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 23 CONFIDENTIAL

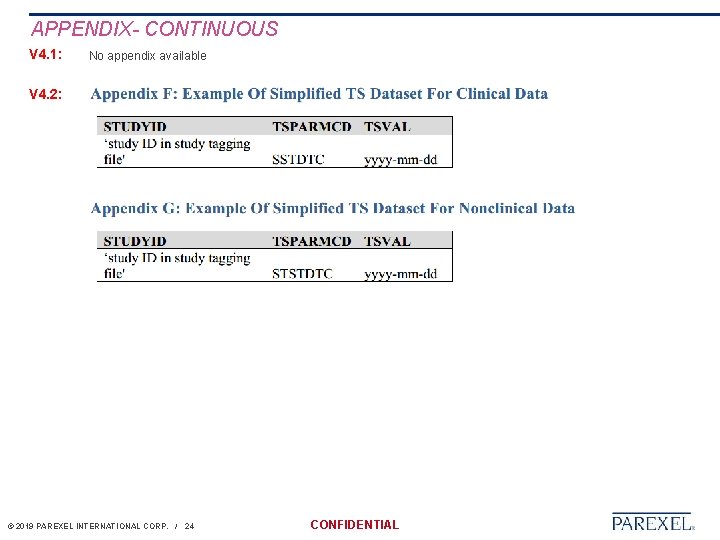
APPENDIX- CONTINUOUS V 4. 1: No appendix available V 4. 2: © 2019 PAREXEL INTERNATIONAL CORP. / 24 CONFIDENTIAL

THANK YOU © 2019 PAREXEL INTERNATIONAL CORP. / 25 CONFIDENTIAL