Chang Pui Chung Memorial School Miss Soo May






















- Slides: 35


Chang Pui Chung Memorial School Miss Soo May Kei Target group: S 6 Student


Background knowledge: 1) Acid-base titration 2) Chemical equilibrium (titration curve) Making Your Own Acid-Base Indicator


• Date: 3 July, 2006 (3 hours) • Searching information and planning investigation • Writing the first page of proposal form Making Your Own Acid-Base Indicator

• Date: 6 July, 2006 (4 hours) • Discussing the first page of the proposal form with the students • Writing the proposal Making Your Own Acid-Base Indicator

• Date: 17 & 18 July, 2006 (6 hours) • Conducting the investigation 1) Making natural indicators 2) Choosing a suitable indicators for titration 3) Determining the concentration of oven cleaner Making Your Own Acid-Base Indicator

• Date: 19 July, 2006 (4 hours) • Organizing and analyzing data Making Your Own Acid-Base Indicator

• Date: 21 July, 2006 (3 hours) • Presenting the findings Making Your Own Acid-Base Indicator

END

How to extract the pigments from the plant materials? For cherries and grapes: Remove their peels For red cabbage, beet root and rose petal: Cut them into small pieces

• Add some water to them and heat the mixtures using a heater

• Filter the solutions using pieces of filter paper

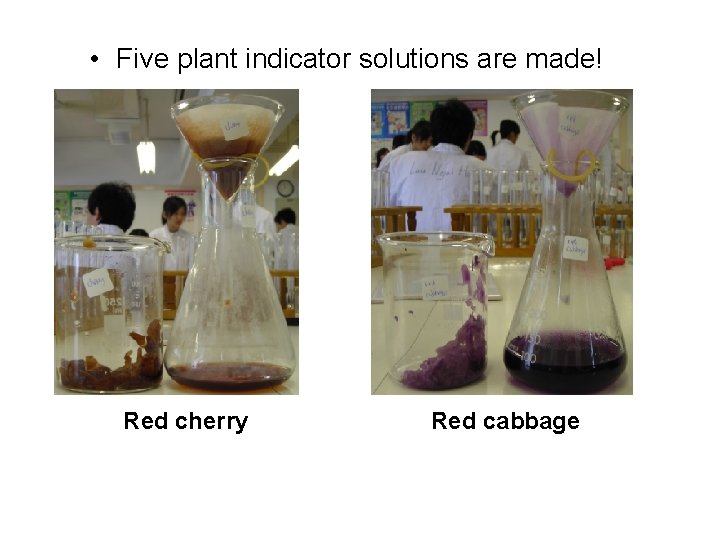
• Five plant indicator solutions are made! Red cherry Red cabbage

Beet root Red grape

Red rose petal Five natural indicators Back

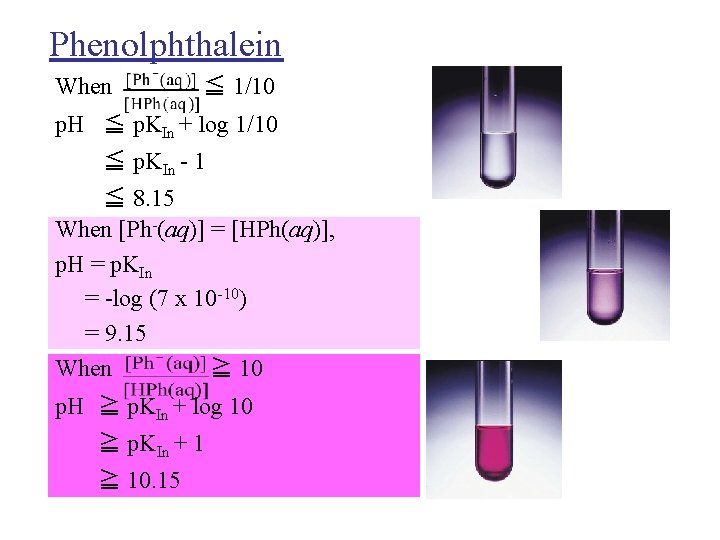
Phenolphthalein When ≦ 1/10 p. H ≦ p. KIn + log 1/10 ≦ p. KIn - 1 ≦ 8. 15 When [Ph-(aq)] = [HPh(aq)], p. H = p. KIn = -log (7 x 10 -10) = 9. 15 When ≧ 10 p. H ≧ p. KIn + log 10 ≧ p. KIn + 1 ≧ 10. 15

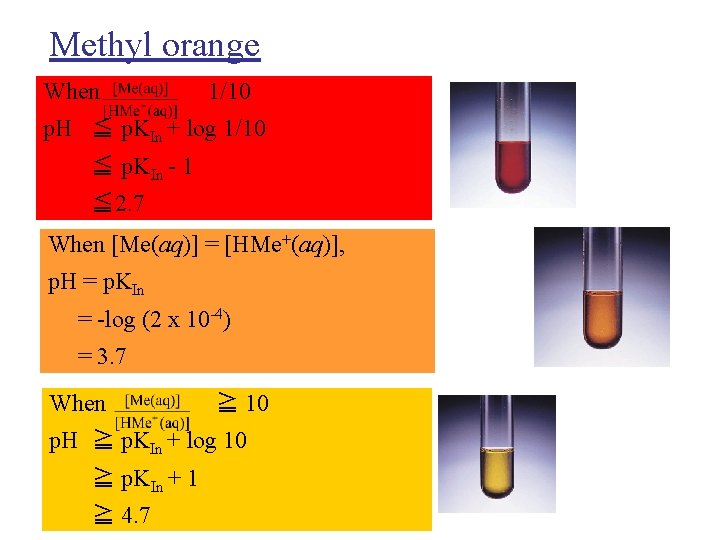
Methyl orange When 1/10 p. H ≦ p. KIn + log 1/10 ≦ p. KIn - 1 ≦ 2. 7 When [Me(aq)] = [HMe+(aq)], p. H = p. KIn = -log (2 x 10 -4) = 3. 7 When ≧ 10 p. H ≧ p. KIn + log 10 ≧ p. KIn + 1 ≧ 4. 7

Acid-base Titrations Acid (CH 3 COOH or HCl) Oven cleaner (Na. OH) + indicator

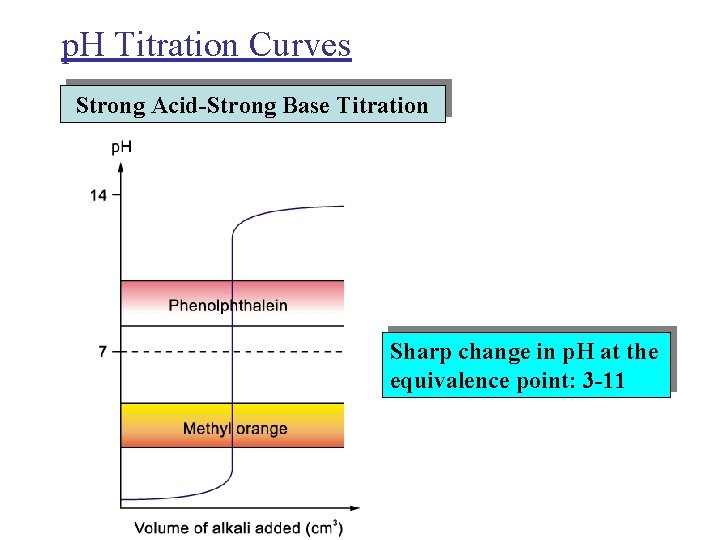
p. H Titration Curves Strong Acid-Strong Base Titration Sharp change in p. H at the equivalence point: 3 -11

Strong Acid-Weak Base Titration Sharp change in p. H at the equivalence point: 2 -6

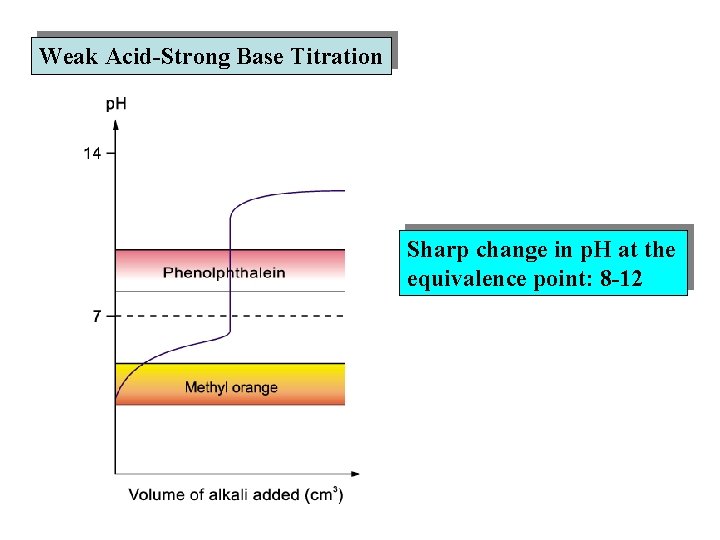
Weak Acid-Strong Base Titration Sharp change in p. H at the equivalence point: 8 -12

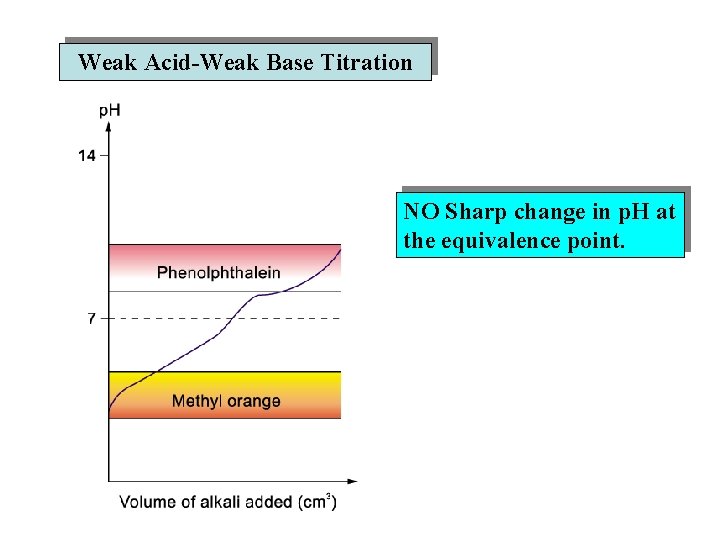
Weak Acid-Weak Base Titration NO Sharp change in p. H at the equivalence point.

Results • Red cabbage showed different colours at different p. H solutions. p. H 12 p. H 2

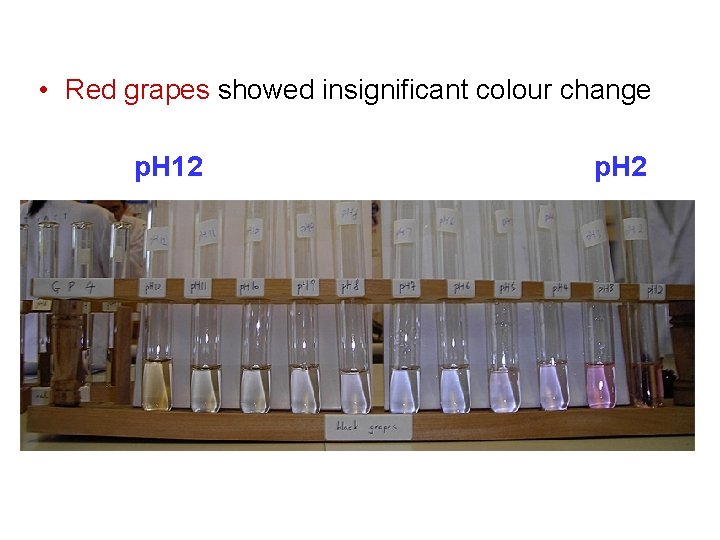
• Red grapes showed insignificant colour change p. H 12 p. H 2

• Red cherries also showed insignificant colour change. p. H 12 p. H 2

• Red rose petals showed a lot of colour at different p. H solution but less significant than red cabbage. p. H 12 p. H 2

• Beet root showed a sharp colour change between p. H 12 and p. H 11 p. H 12 p. H 2

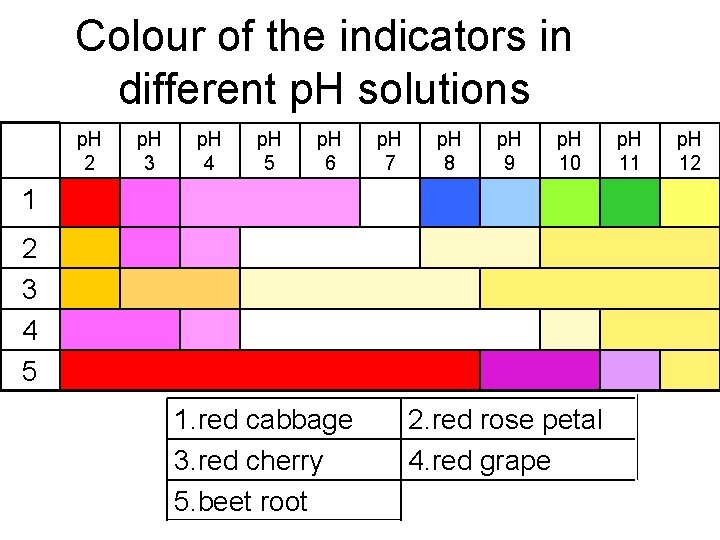
Colour of the indicators in different p. H solutions p. H 2 p. H 3 p. H 4 p. H 5 p. H 6 p. H 7 p. H 8 p. H 9 p. H 10 1 2 3 4 5 1. red cabbage 3. red cherry 5. beet root 2. red rose petal 4. red grape p. H 11 p. H 12

• Beet root showed a sharp colour change between p. H 12 and p. H 11 p. H 12 p. H 2 As it showed a sharp colour change at alkaline region, it is suitable for being used as indicator in acid-base titration. Back

Freshly prepared beet root solution p. H 12 p. H 2 Overnight beet root solution p. H 12 p. H 2

Colour of the indicators (overnight and fresh beet root solution)in different p. H solutions p. H p. H 2 3 4 5 6 7 8 9 10 11 12 1 2 1. Overnight 2. Fresh

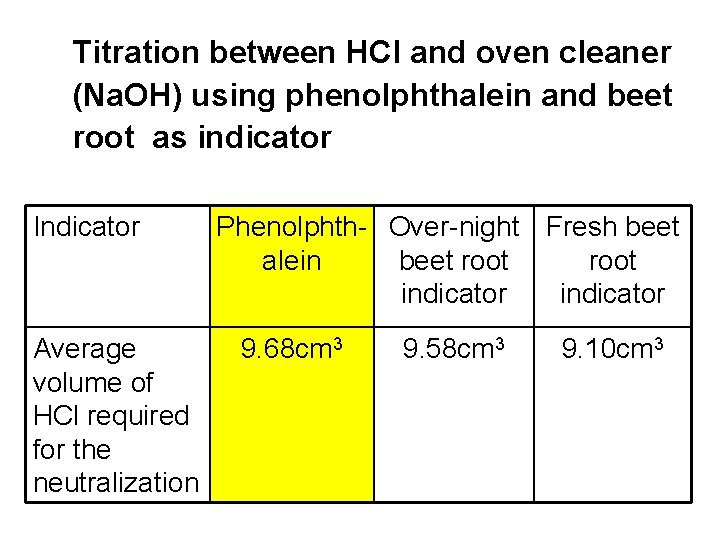
Titration between HCl and oven cleaner (Na. OH) using phenolphthalein and beet root as indicator Indicator Average volume of HCl required for the neutralization Phenolphth- Over-night Fresh beet alein beet root indicator 9. 68 cm 3 9. 58 cm 3 9. 10 cm 3

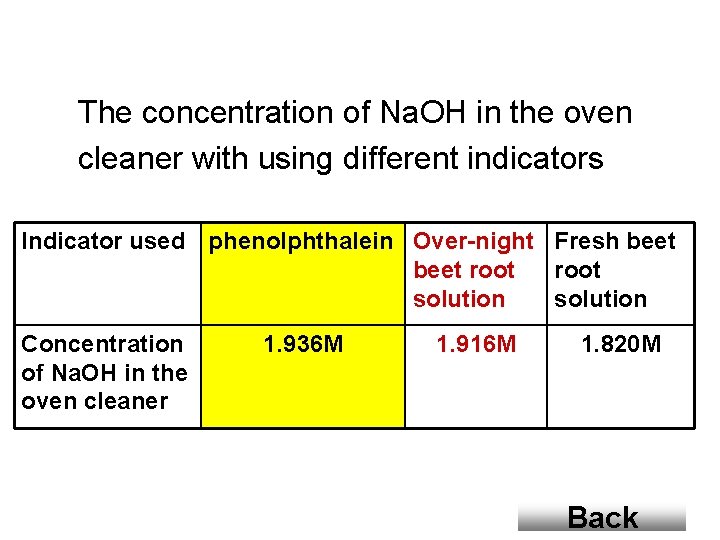
The concentration of Na. OH in the oven cleaner with using different indicators Indicator used phenolphthalein Over-night Fresh beet root solution Concentration of Na. OH in the oven cleaner 1. 936 M 1. 916 M 1. 820 M Back
 Chang pui chung memorial school
Chang pui chung memorial school Chúng tôi đứng trên núi chung
Chúng tôi đứng trên núi chung Chớ mang ách chung với kẻ chẳng tin
Chớ mang ách chung với kẻ chẳng tin Pui tak canossian primary school
Pui tak canossian primary school Chẳng may em đánh vỡ một lọ hoa đẹp
Chẳng may em đánh vỡ một lọ hoa đẹp Ethan soo
Ethan soo Tan soo suan
Tan soo suan Soo-siang lim
Soo-siang lim Dr ida soo
Dr ida soo Park bong
Park bong Vrf cisco configuration
Vrf cisco configuration Lady macbeth important quotes
Lady macbeth important quotes Soo
Soo Teo soo hwang
Teo soo hwang Broward schools sso
Broward schools sso Chung hua middle school no.4
Chung hua middle school no.4 La joya reading renaissance
La joya reading renaissance Barnhill memorial school
Barnhill memorial school William knibb memorial high school
William knibb memorial high school Saint malachys
Saint malachys Manchester memorial elementary school
Manchester memorial elementary school The mustang way
The mustang way Olcott memorial higher secondary school
Olcott memorial higher secondary school Satish chandra memorial school
Satish chandra memorial school Olcott memorial high school
Olcott memorial high school Warna hijau pada lambang pui melambangkan
Warna hijau pada lambang pui melambangkan Intisab adalah
Intisab adalah Tui cui
Tui cui Creang
Creang Prin ce se înmulțesc animalele
Prin ce se înmulțesc animalele Lambang lambang roh kudus
Lambang lambang roh kudus Simbol univet
Simbol univet Pui
Pui Please just miss once
Please just miss once Uil ilpc
Uil ilpc Hci patterns
Hci patterns