Challenge 1 T Trimpe 2008 http sciencespot net






- Slides: 11

Challenge 1 T. Trimpe 2008 http: //sciencespot. net/

Use a periodic table and your knowledge of the element families to identify each element by name. 1. I am a member of the Carbon family with 14 electrons. 2. I am a member of the Alkaline Earth family and would have 6 energy levels. 3. I am a halide that is a liquid at room temperature. 4. I am a transition metal with 79 protons. 5. I am a member of the Alkali Metal family with 20 neutrons.

The answers are … 1. I am a member of the Carbon family with 14 electrons. Silicon 2. I am a member of the Alkaline Earth family and would have 6 energy levels. Barium 3. I am a halide that is a liquid at room temperature. Bromine 4. I am a transition metal with 79 protons. Gold 5. I am a member of the Alkali Metal family with 20 neutrons. Potassium

Use a periodic table and your knowledge of the element families to identify each element by name. 1. I am a member of the Halide family and am a liquid at room temperature. 2. I am a member of the Alkaline Earth family and would have 4 energy levels. 3. I am a member of the Nitrogen family that is gas at room temperature. 4. I am a transition metal with 110 neutrons. 5. I am a member of the Oxygen family with 16 protons and 16 neutrons.

The answers are … 1. I am a member of the Boron family and am a liquid at room temperature. Bromine 2. I am a member of the Alkaline Earth family and would have 4 energy levels. Calcium 3. I am a member of the Nitrogen family that is gas at room temperature. Nitrogen 4. I am a transition metal with 110 neutrons. Tungsten 5. I am a member of the Oxygen family with 16 protons and 16 neutrons. Sulfur

Use a periodic table and your knowledge of the element families to identify each element by name. 1. I am a Noble Gas that is found in the 6 th period. 2. I am a member of the Alkaline Earth family and have 88 electrons. 3. I am a transition metal that is a liquid at room temperature. 4. I am a member of the Carbon family that is found in the 5 th period. 5. I am a Halide that is a gas at room temperature and have 3 energy levels.

The answers are … 1. I am a Noble Gas that is found in the 6 th period. Radon 2. I am a member of the Alkaline Earth family and have 88 electrons. Radium 3. I am a transition metal that is a liquid at room temperature. Mercury 4. I am a member of the Carbon family that is found in the 5 th period. 5. I am a Halide that is a gas at room temperature and have 3 energy levels. Tin Chlorine

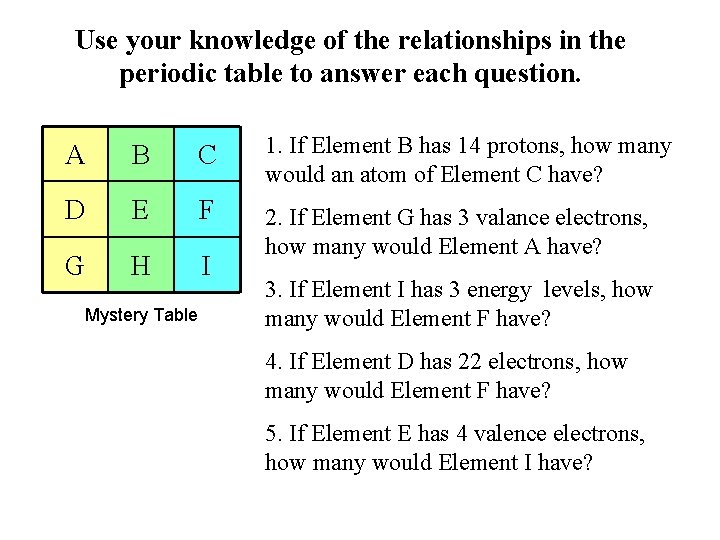
Use your knowledge of the relationships in the periodic table to answer each question. A B C 1. If Element B has 14 protons, how many would an atom of Element C have? D E F G H I 2. If Element G has 3 valance electrons, how many would Element A have? Mystery Table 3. If Element I has 3 energy levels, how many would Element F have? 4. If Element D has 22 electrons, how many would Element F have? 5. If Element E has 4 valence electrons, how many would Element I have?

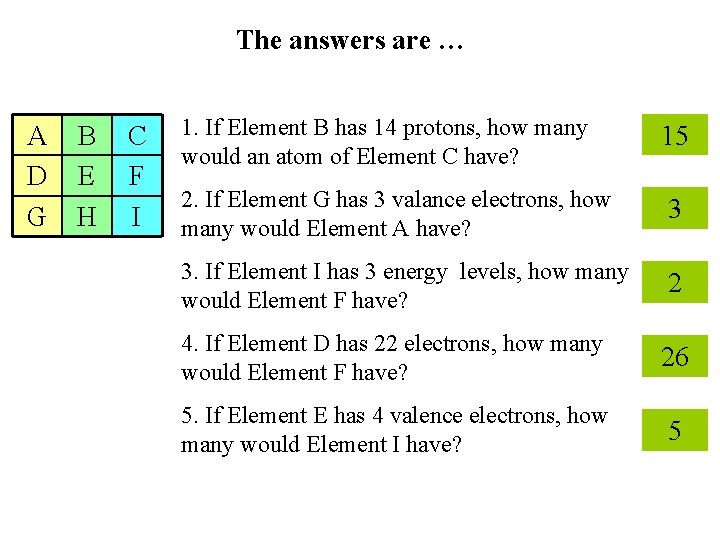
The answers are … A D G B E H C F I 1. If Element B has 14 protons, how many would an atom of Element C have? 15 2. If Element G has 3 valance electrons, how many would Element A have? 3 3. If Element I has 3 energy levels, how many would Element F have? 2 4. If Element D has 22 electrons, how many would Element F have? 26 5. If Element E has 4 valence electrons, how many would Element I have? 5

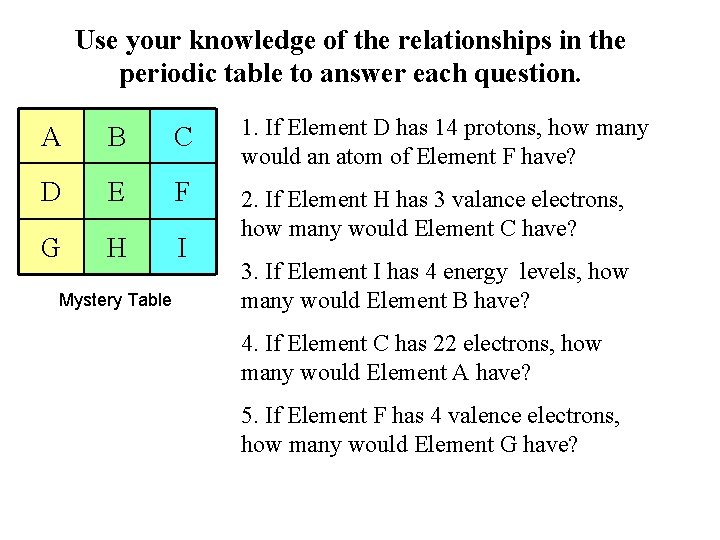
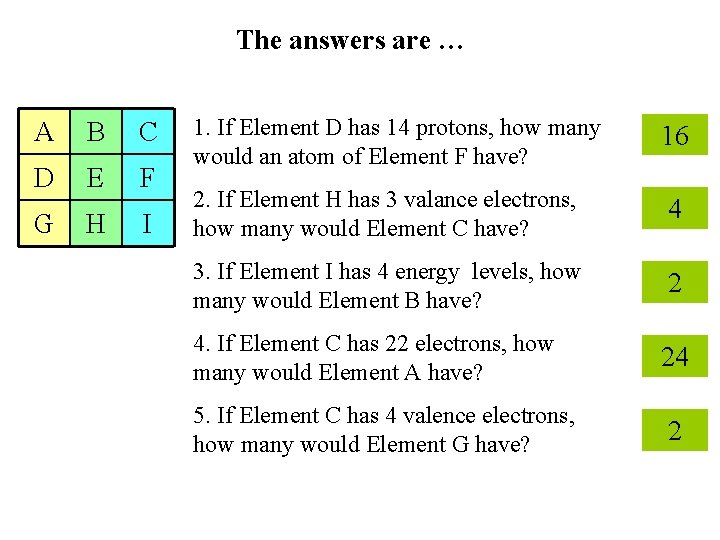
Use your knowledge of the relationships in the periodic table to answer each question. A B C 1. If Element D has 14 protons, how many would an atom of Element F have? D E F G H I 2. If Element H has 3 valance electrons, how many would Element C have? Mystery Table 3. If Element I has 4 energy levels, how many would Element B have? 4. If Element C has 22 electrons, how many would Element A have? 5. If Element F has 4 valence electrons, how many would Element G have?

The answers are … A B C D E F G H I 1. If Element D has 14 protons, how many would an atom of Element F have? 16 2. If Element H has 3 valance electrons, how many would Element C have? 4 3. If Element I has 4 energy levels, how many would Element B have? 2 4. If Element C has 22 electrons, how many would Element A have? 24 5. If Element C has 4 valence electrons, how many would Element G have? 2