Ch 9 Gas Separation by Membranes Separation of
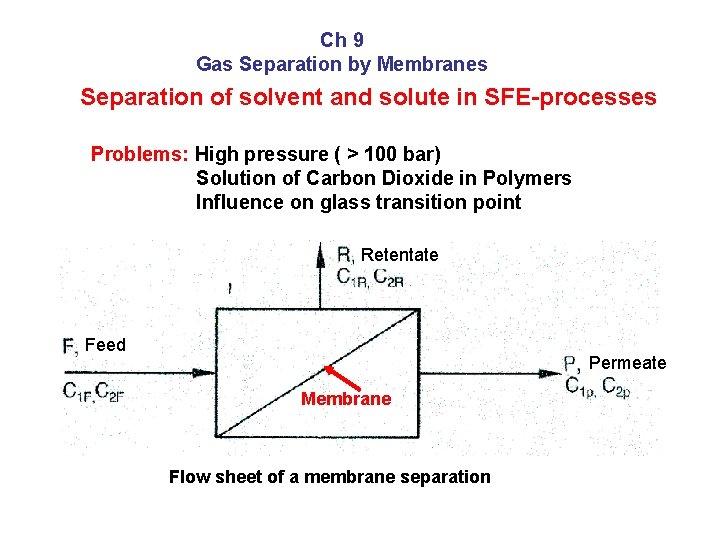
Ch 9 Gas Separation by Membranes Separation of solvent and solute in SFE-processes Problems: High pressure ( > 100 bar) Solution of Carbon Dioxide in Polymers Influence on glass transition point Retentate Feed Permeate Membrane Flow sheet of a membrane separation
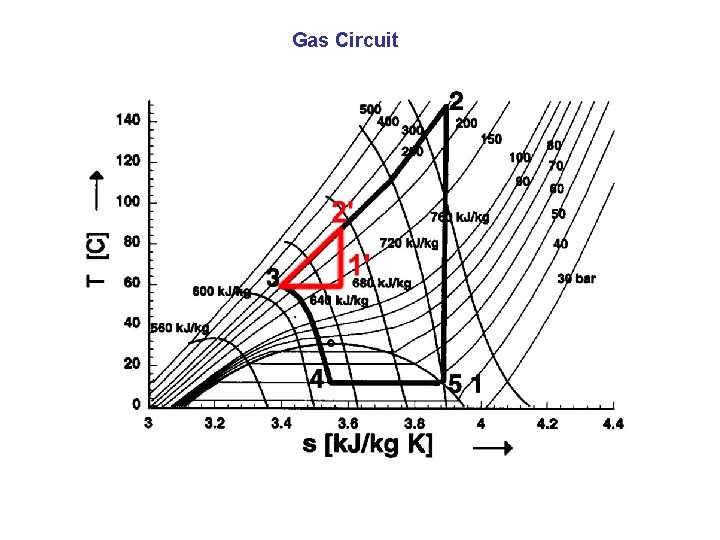
Gas Circuit
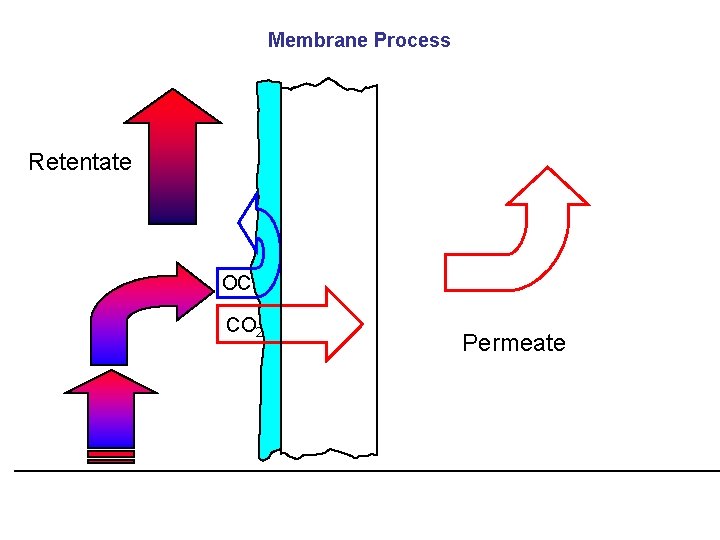
Membrane Process Retentate OC CO 2 Permeate
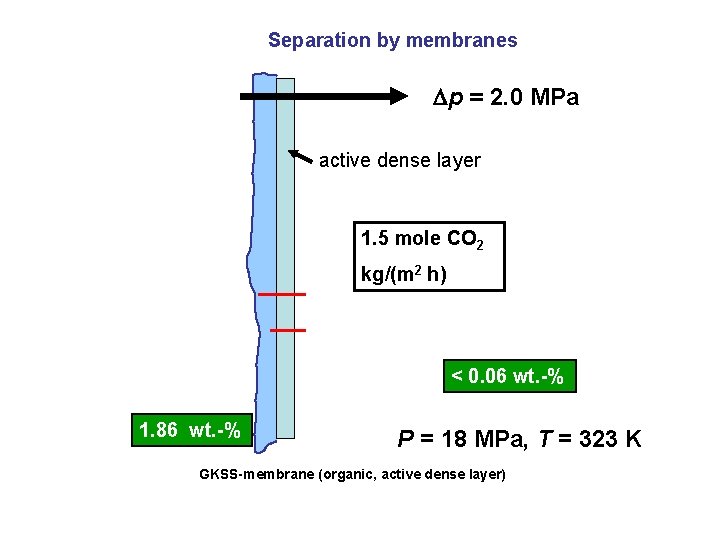
Separation by membranes p = 2. 0 MPa active dense layer 1. 5 mole CO 2 kg/(m 2 h) < 0. 06 wt. -% 1. 86 wt. -% P = 18 MPa, T = 323 K GKSS-membrane (organic, active dense layer)
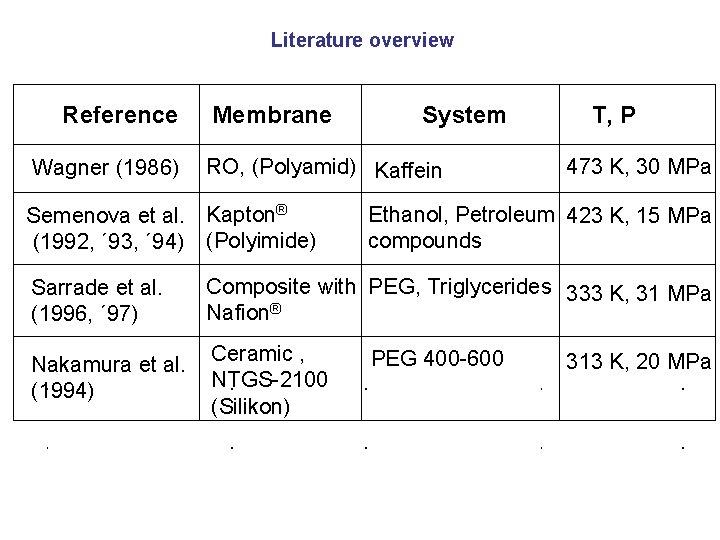
Literature overview Reference Wagner (1986) Membrane RO, (Polyamid) Kaffein Semenova et al. Kapton® (1992, ´ 93, ´ 94) (Polyimide) Sarrade et al. (1996, ´ 97) System T, P 473 K, 30 MPa Ethanol, Petroleum 423 K, 15 MPa compounds Composite with PEG, Triglycerides 333 K, 31 MPa Nafion® Nakamura et al. Ceramic , NTGS-2100 (1994) (Silikon) PEG 400 -600 313 K, 20 MPa
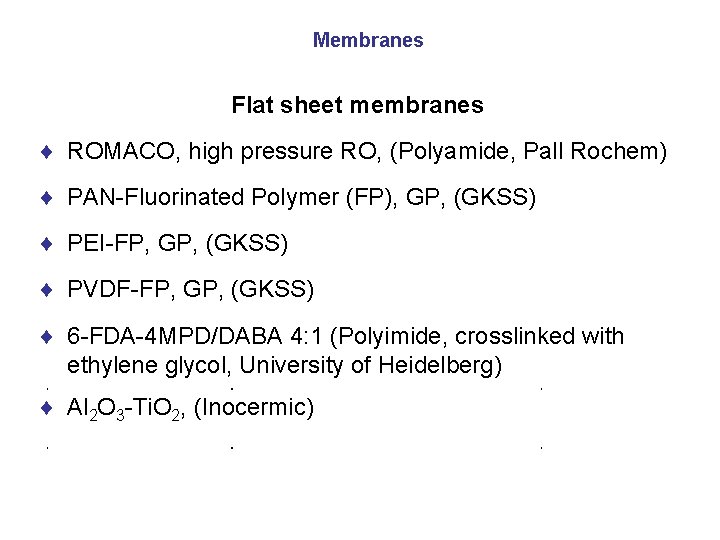
Membranes Flat sheet membranes ¨ ROMACO, high pressure RO, (Polyamide, Pall Rochem) ¨ PAN-Fluorinated Polymer (FP), GP, (GKSS) ¨ PEI-FP, GP, (GKSS) ¨ PVDF-FP, GP, (GKSS) ¨ 6 -FDA-4 MPD/DABA 4: 1 (Polyimide, crosslinked with ethylene glycol, University of Heidelberg) ¨ Al 2 O 3 -Ti. O 2, (Inocermic)
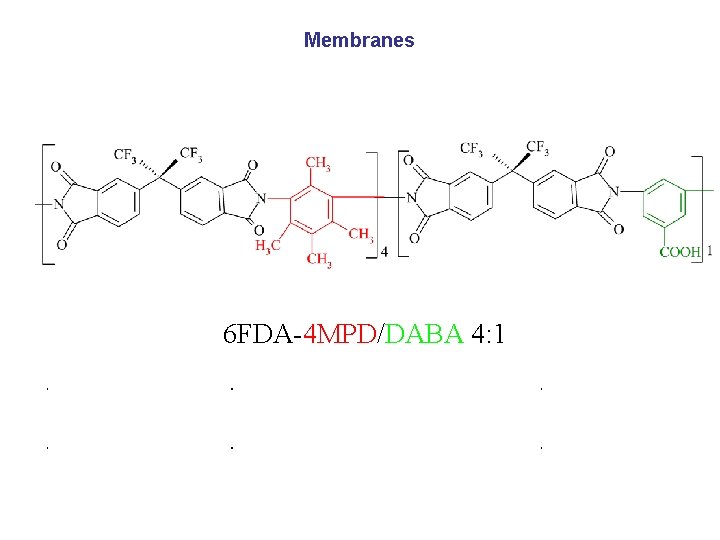
Membranes 6 FDA-4 MPD/DABA 4: 1
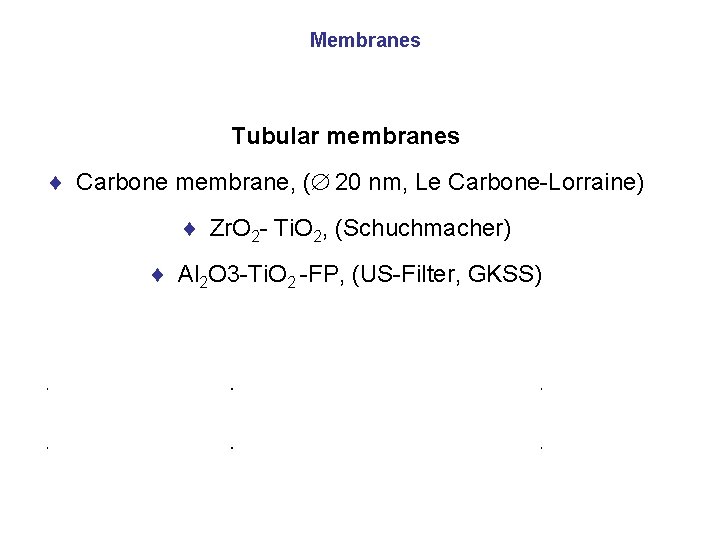
Membranes Tubular membranes ¨ Carbone membrane, ( 20 nm, Le Carbone-Lorraine) ¨ Zr. O 2 - Ti. O 2, (Schuchmacher) ¨ Al 2 O 3 -Ti. O 2 -FP, (US-Filter, GKSS)

Membranes Mechanisms of membrane transport
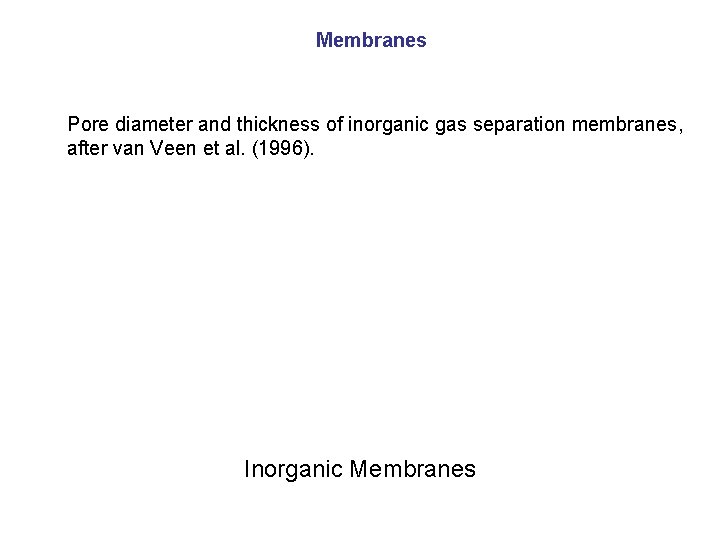
Membranes Pore diameter and thickness of inorganic gas separation membranes, after van Veen et al. (1996). Inorganic Membranes

Membranes Classification of ceramic membranes (Bonekamp, 1996).
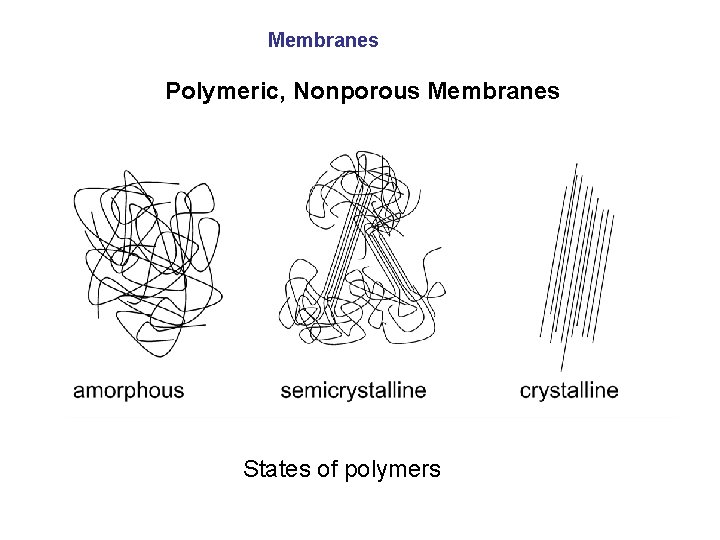
Membranes Polymeric, Nonporous Membranes States of polymers
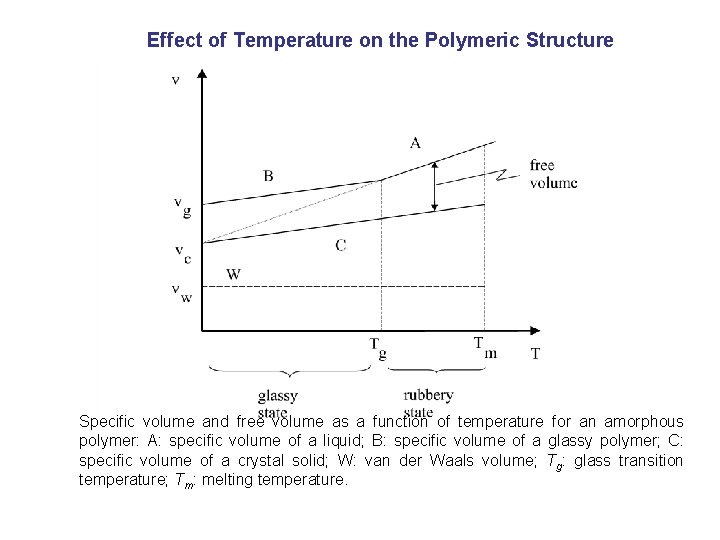
Effect of Temperature on the Polymeric Structure Specific volume and free volume as a function of temperature for an amorphous polymer: A: specific volume of a liquid; B: specific volume of a glassy polymer; C: specific volume of a crystal solid; W: van der Waals volume; Tg: glass transition temperature; Tm: melting temperature.
Membranes Influences on Membrane Properties Effect of Temperature on the Polymeric Structure Effect of Pressure on the Polymeric Structure Swelling and Plasticization of Polymers Aging of Polymers
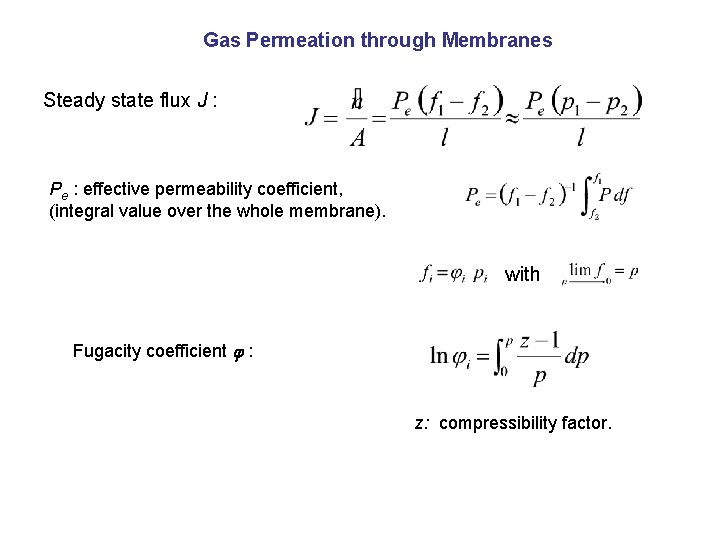
Gas Permeation through Membranes Steady state flux J : Pe : effective permeability coefficient, (integral value over the whole membrane). with Fugacity coefficient : z: compressibility factor.
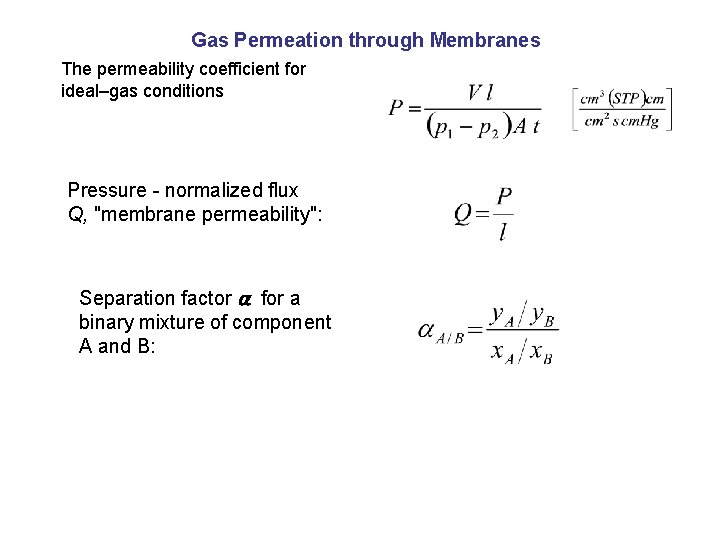
Gas Permeation through Membranes The permeability coefficient for ideal–gas conditions Pressure - normalized flux Q, "membrane permeability": Separation factor for a binary mixture of component A and B:
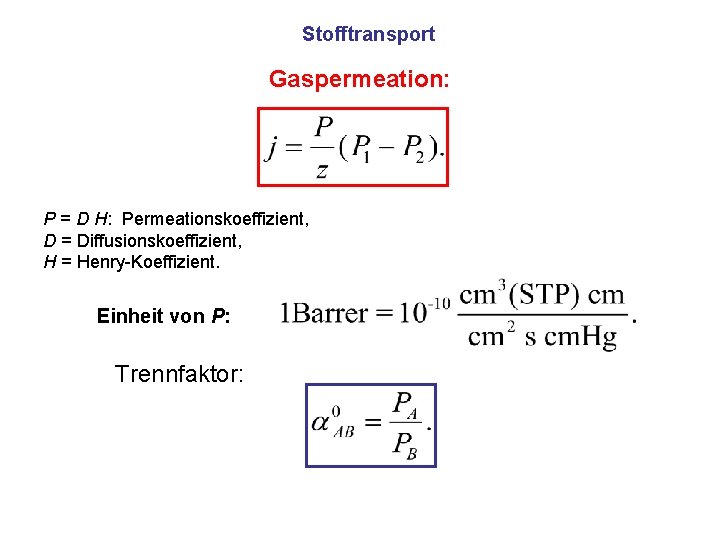
Stofftransport Gaspermeation: P = D H: Permeationskoeffizient, D = Diffusionskoeffizient, H = Henry-Koeffizient. Einheit von P: Trennfaktor:
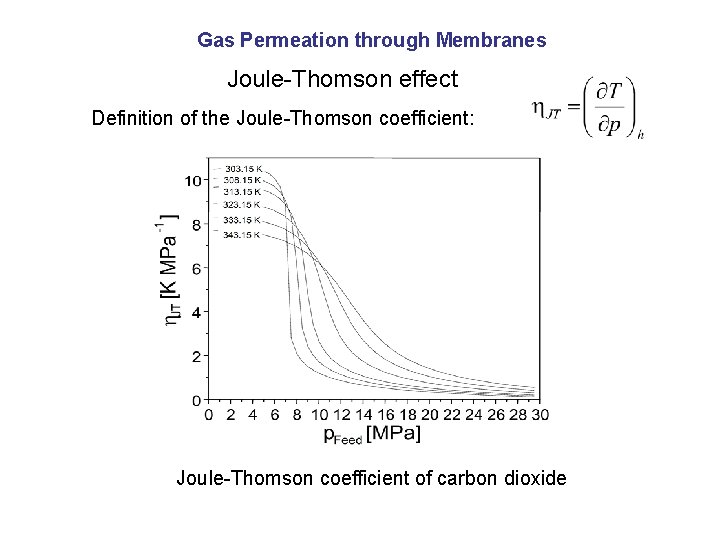
Gas Permeation through Membranes Joule-Thomson effect Definition of the Joule-Thomson coefficient: Joule-Thomson coefficient of carbon dioxide
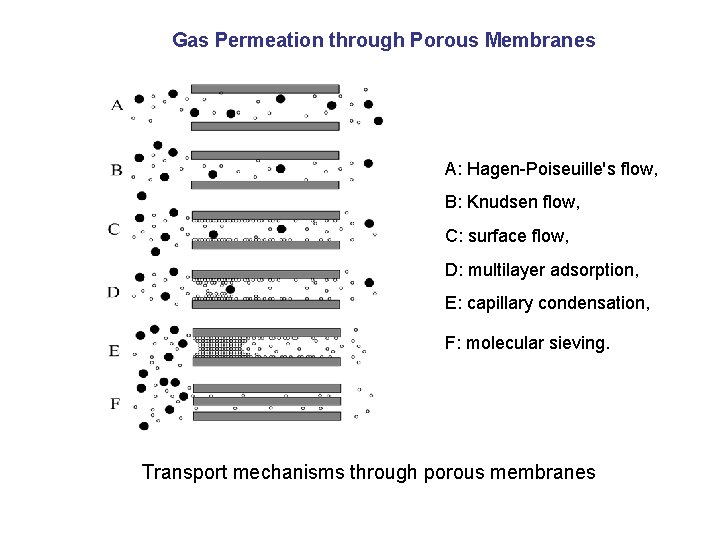
Gas Permeation through Porous Membranes A: Hagen-Poiseuille's flow, B: Knudsen flow, C: surface flow, D: multilayer adsorption, E: capillary condensation, F: molecular sieving. Transport mechanisms through porous membranes
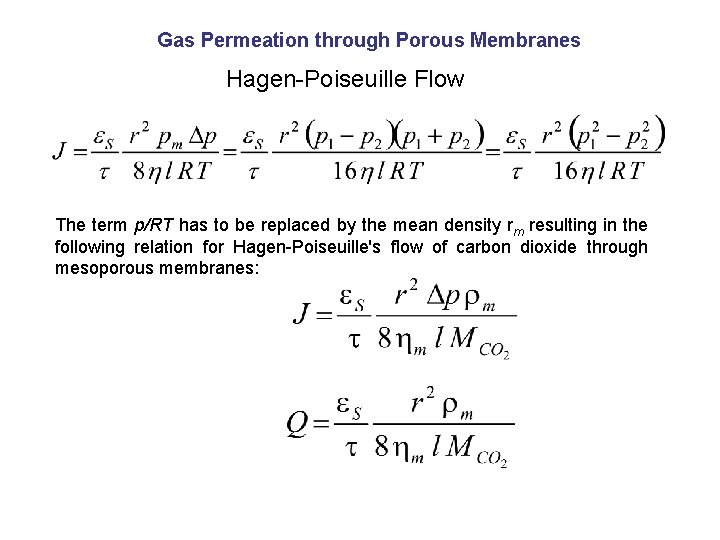
Gas Permeation through Porous Membranes Hagen-Poiseuille Flow The term p/RT has to be replaced by the mean density rm resulting in the following relation for Hagen-Poiseuille's flow of carbon dioxide through mesoporous membranes:
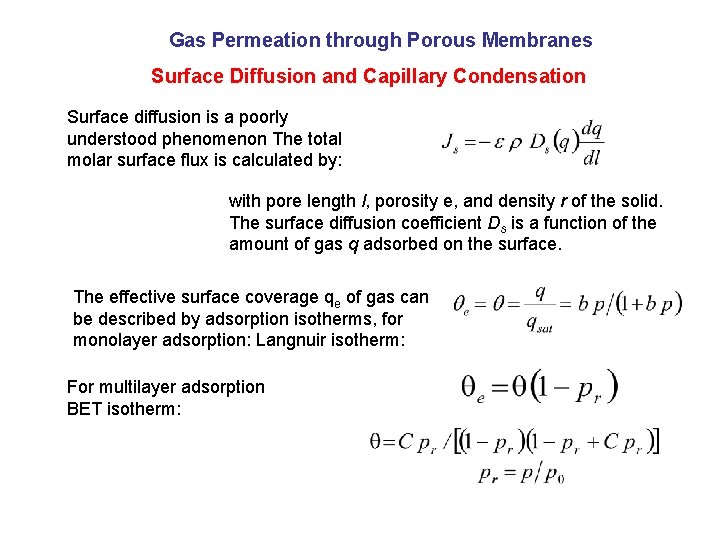
Gas Permeation through Porous Membranes Surface Diffusion and Capillary Condensation Surface diffusion is a poorly understood phenomenon The total molar surface flux is calculated by: with pore length l, porosity e, and density r of the solid. The surface diffusion coefficient Ds is a function of the amount of gas q adsorbed on the surface. The effective surface coverage qe of gas can be described by adsorption isotherms, for monolayer adsorption: Langnuir isotherm: For multilayer adsorption BET isotherm:
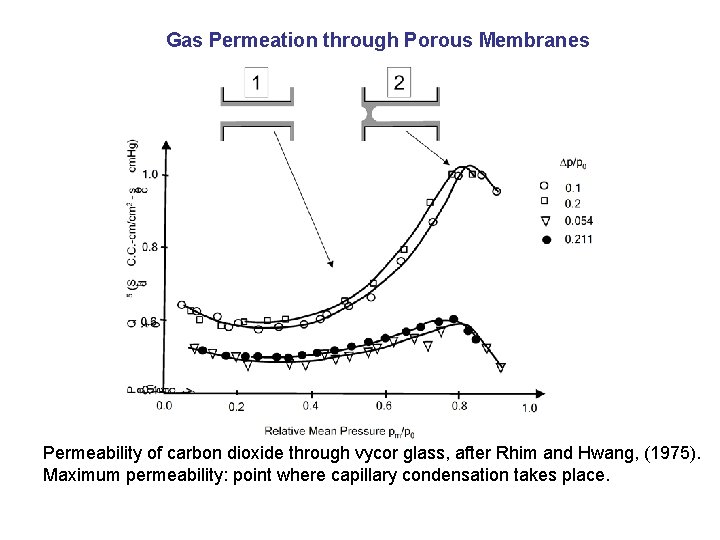
Gas Permeation through Porous Membranes Permeability of carbon dioxide through vycor glass, after Rhim and Hwang, (1975). Maximum permeability: point where capillary condensation takes place.
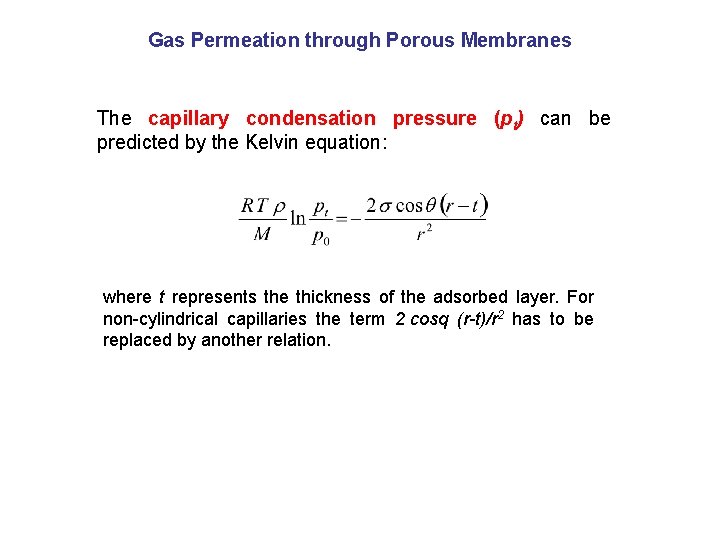
Gas Permeation through Porous Membranes The capillary condensation pressure (pt) can be predicted by the Kelvin equation: where t represents the thickness of the adsorbed layer. For non-cylindrical capillaries the term 2 cosq (r-t)/r 2 has to be replaced by another relation.
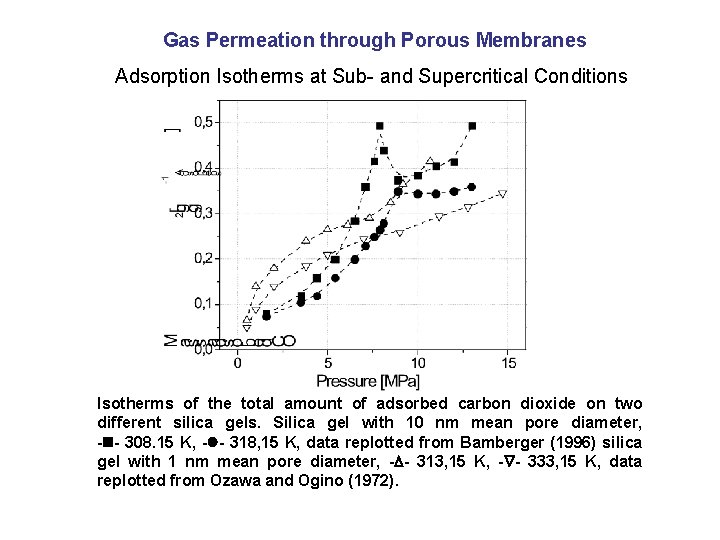
Gas Permeation through Porous Membranes Adsorption Isotherms at Sub- and Supercritical Conditions Isotherms of the total amount of adsorbed carbon dioxide on two different silica gels. Silica gel with 10 nm mean pore diameter, - - 308. 15 K, - - 318, 15 K, data replotted from Bamberger (1996) silica gel with 1 nm mean pore diameter, - - 313, 15 K, - - 333, 15 K, data replotted from Ozawa and Ogino (1972).
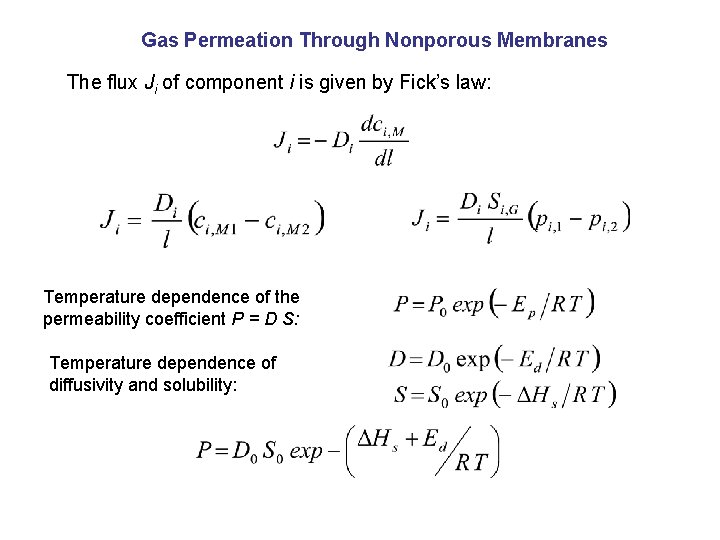
Gas Permeation Through Nonporous Membranes The flux Ji of component i is given by Fick’s law: Temperature dependence of the permeability coefficient P = D S: Temperature dependence of diffusivity and solubility:
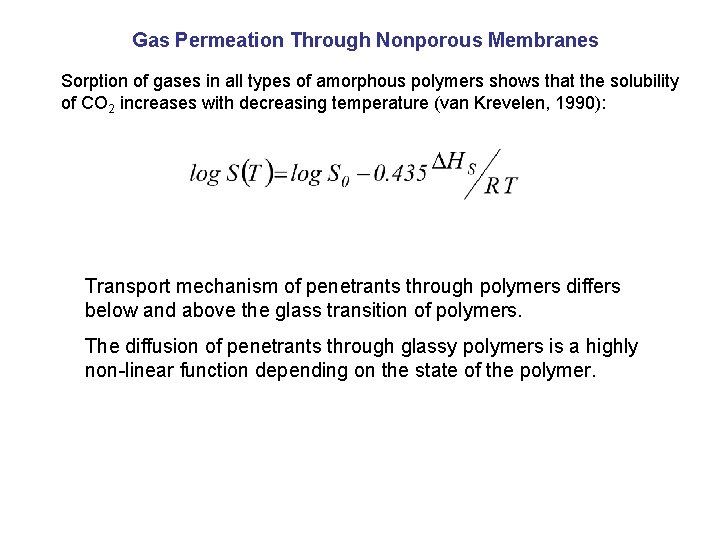
Gas Permeation Through Nonporous Membranes Sorption of gases in all types of amorphous polymers shows that the solubility of CO 2 increases with decreasing temperature (van Krevelen, 1990): Transport mechanism of penetrants through polymers differs below and above the glass transition of polymers. The diffusion of penetrants through glassy polymers is a highly non-linear function depending on the state of the polymer.
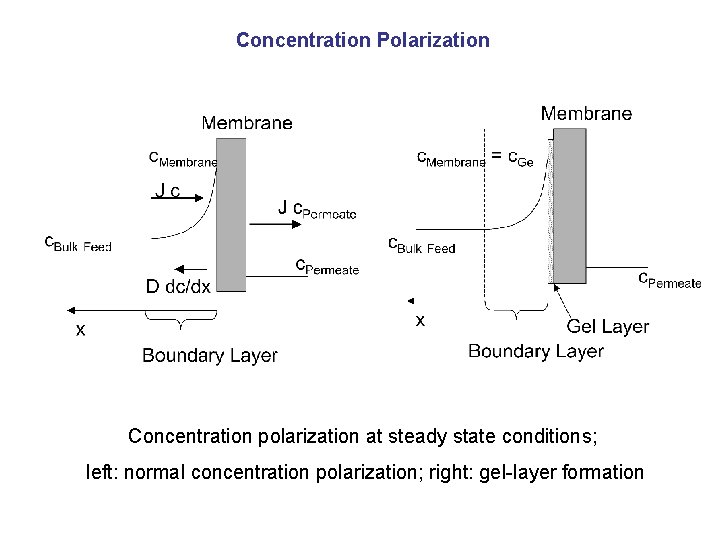
Concentration Polarization Concentration polarization at steady state conditions; left: normal concentration polarization; right: gel-layer formation
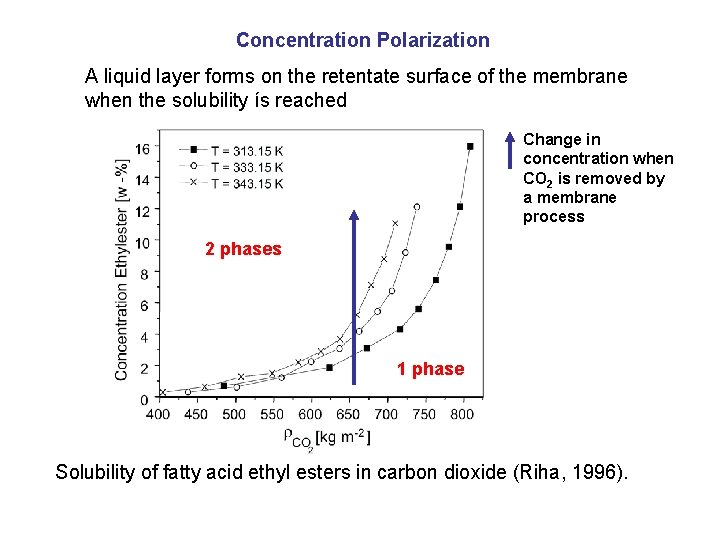
Concentration Polarization A liquid layer forms on the retentate surface of the membrane when the solubility ís reached Change in concentration when CO 2 is removed by a membrane process 2 phases 1 phase Solubility of fatty acid ethyl esters in carbon dioxide (Riha, 1996).
Membrane Test Cell Membrane

Flat Sheet Test Cell
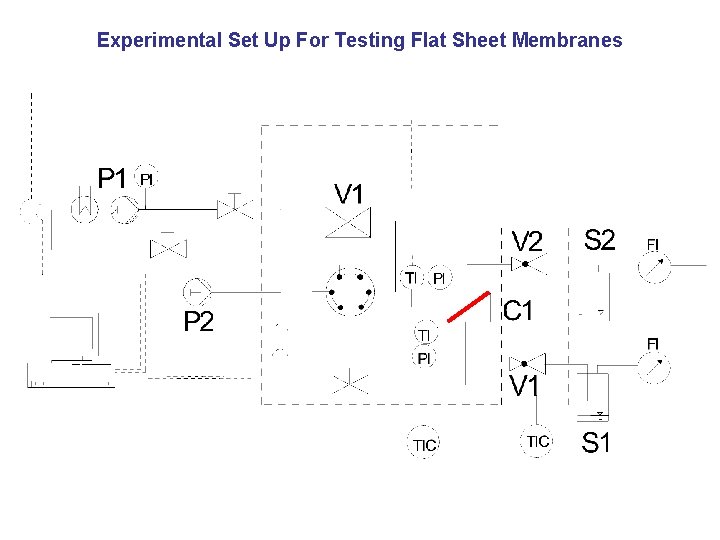
Experimental Set Up For Testing Flat Sheet Membranes
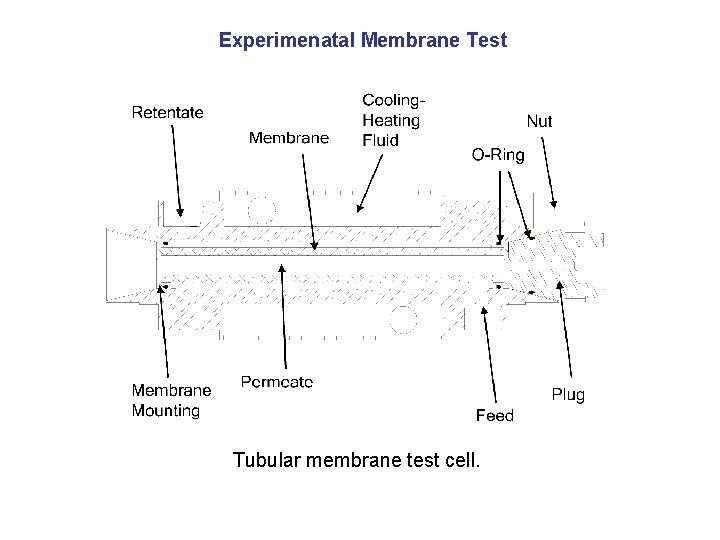
Experimenatal Membrane Test Tubular membrane test cell.
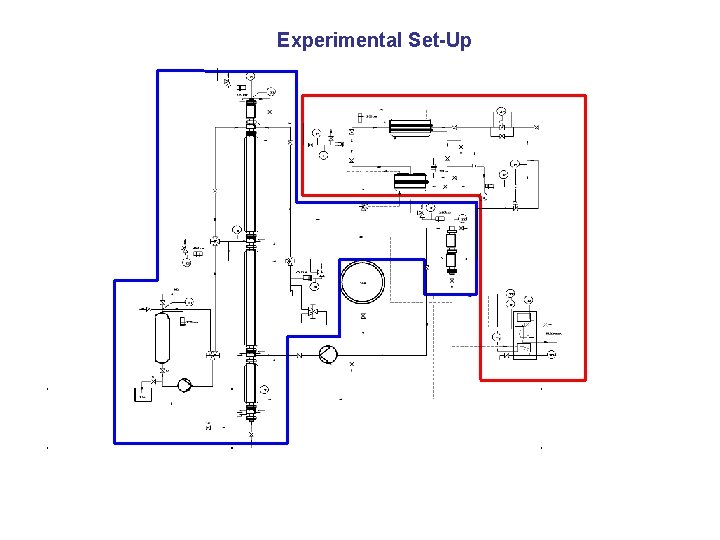
Experimental Set-Up
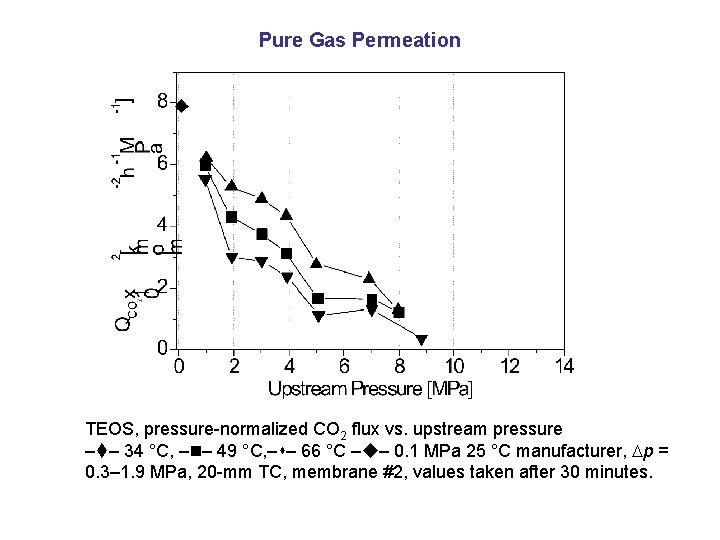
Pure Gas Permeation TEOS, pressure-normalized CO 2 flux vs. upstream pressure – – 34 °C, – – 49 °C, – – 66 °C – – 0. 1 MPa 25 °C manufacturer, p = 0. 3– 1. 9 MPa, 20 -mm TC, membrane #2, values taken after 30 minutes.
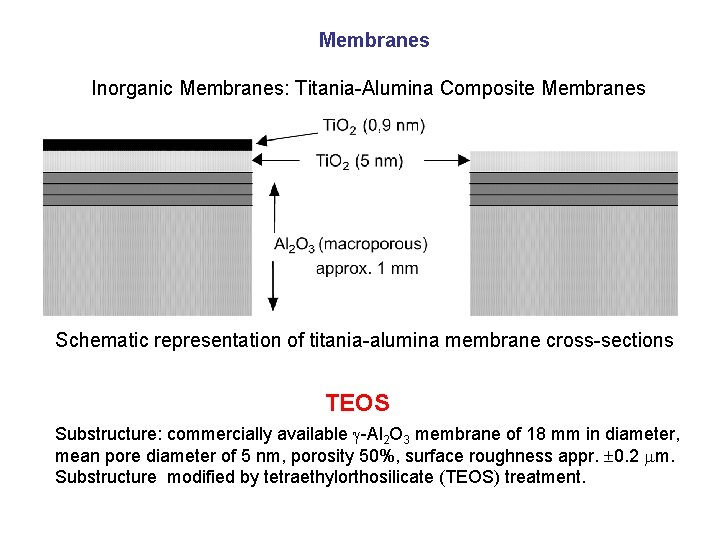
Membranes Inorganic Membranes: Titania-Alumina Composite Membranes Schematic representation of titania-alumina membrane cross-sections TEOS Substructure: commercially available g-Al 2 O 3 membrane of 18 mm in diameter, mean pore diameter of 5 nm, porosity 50%, surface roughness appr. 0. 2 mm. Substructure modified by tetraethylorthosilicate (TEOS) treatment.
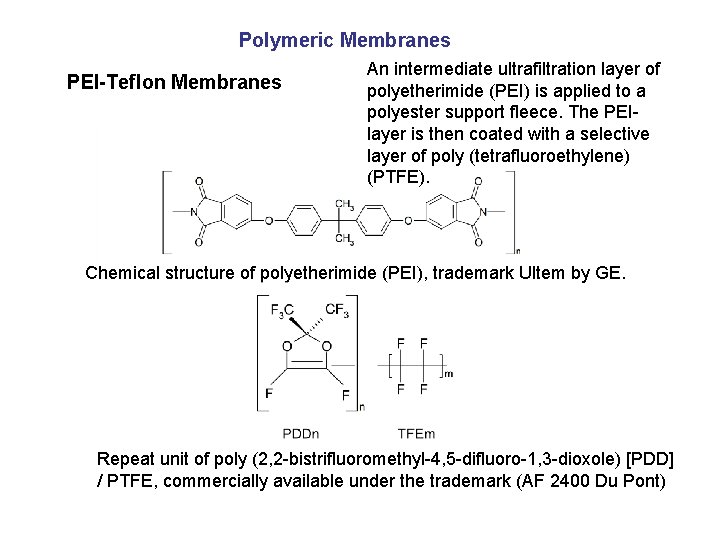
Polymeric Membranes PEI-Teflon Membranes An intermediate ultrafiltration layer of polyetherimide (PEI) is applied to a polyester support fleece. The PEIlayer is then coated with a selective layer of poly (tetrafluoroethylene) (PTFE). Chemical structure of polyetherimide (PEI), trademark Ultem by GE. Repeat unit of poly (2, 2 -bistrifluoromethyl-4, 5 -difluoro-1, 3 -dioxole) [PDD] / PTFE, commercially available under the trademark (AF 2400 Du Pont)
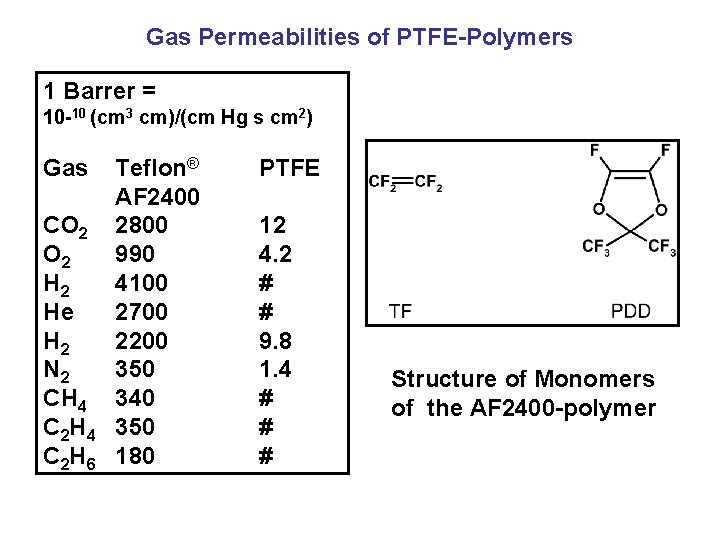
Gas Permeabilities of PTFE-Polymers 1 Barrer = 10 -10 (cm 3 cm)/(cm Hg s cm 2) Gas CO 2 H 2 He H 2 N 2 CH 4 C 2 H 6 Teflon® AF 2400 2800 990 4100 2700 2200 350 340 350 180 PTFE 12 4. 2 # # 9. 8 1. 4 # # # Structure of Monomers of the AF 2400 -polymer
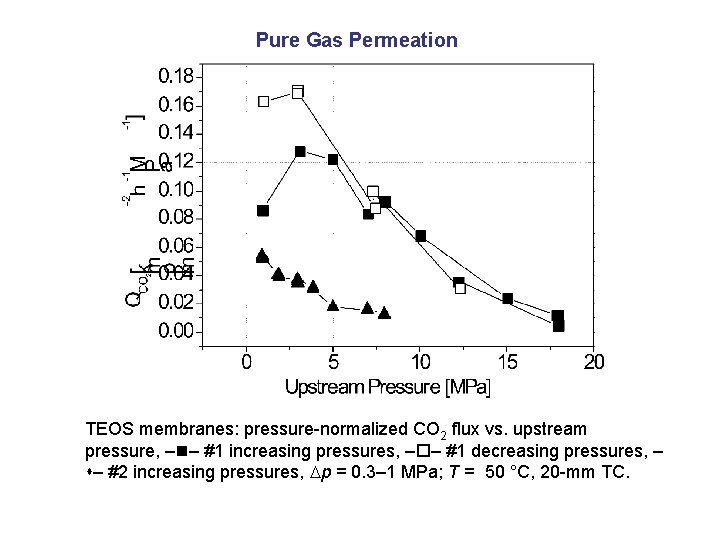
Pure Gas Permeation TEOS membranes: pressure-normalized CO 2 flux vs. upstream pressure, – – #1 increasing pressures, – – #1 decreasing pressures, – – #2 increasing pressures, p = 0. 3– 1 MPa; T = 50 °C, 20 -mm TC.
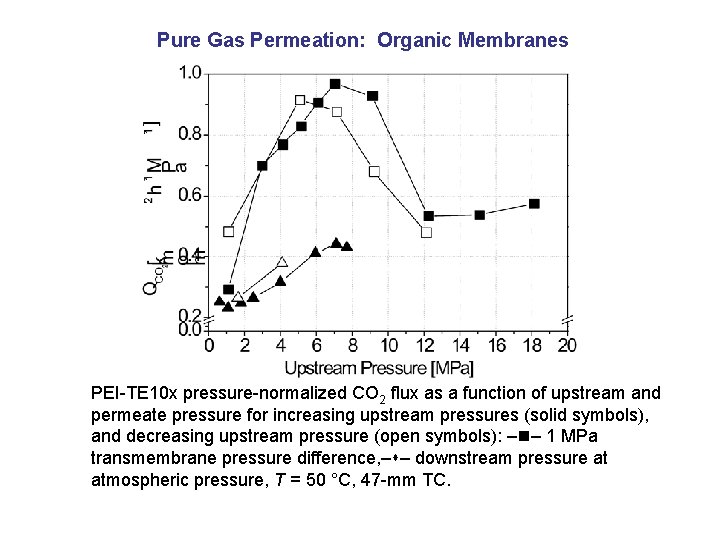
Pure Gas Permeation: Organic Membranes PEI-TE 10 x pressure-normalized CO 2 flux as a function of upstream and permeate pressure for increasing upstream pressures (solid symbols), and decreasing upstream pressure (open symbols): – – 1 MPa transmembrane pressure difference, – – downstream pressure at atmospheric pressure, T = 50 °C, 47 -mm TC.
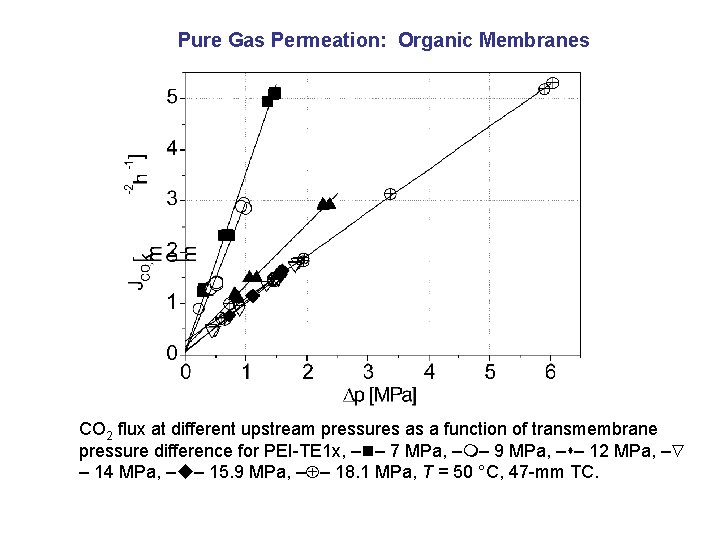
Pure Gas Permeation: Organic Membranes CO 2 flux at different upstream pressures as a function of transmembrane pressure difference for PEI-TE 1 x, – – 7 MPa, – – 9 MPa, – – 12 MPa, – – 14 MPa, – – 15. 9 MPa, – – 18. 1 MPa, T = 50 °C, 47 -mm TC.
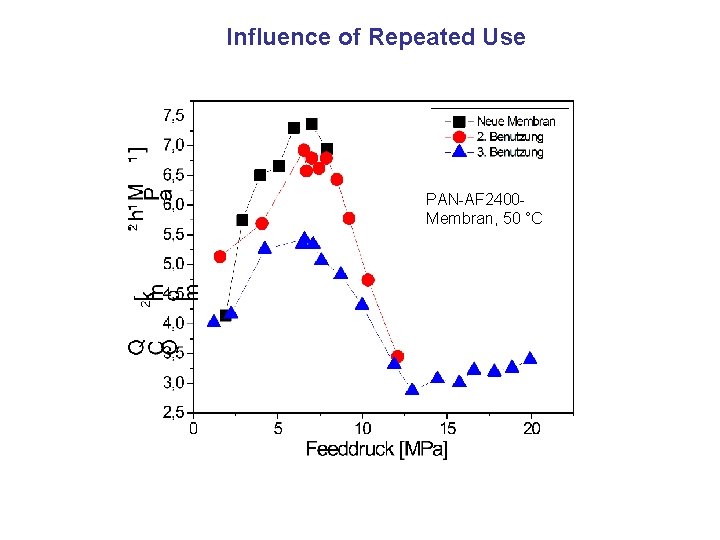
Influence of Repeated Use PAN-AF 2400 Membran, 50 °C

CO 2 -Permeate Flow of PEI-AF 2400 -1 x Related to PEI-AF 2400 -10 x – – increasing retentate Pressure (Feed side), – – decreasing retentate pressure (Feed side), T = 50 °C, 47 -mm test cell.
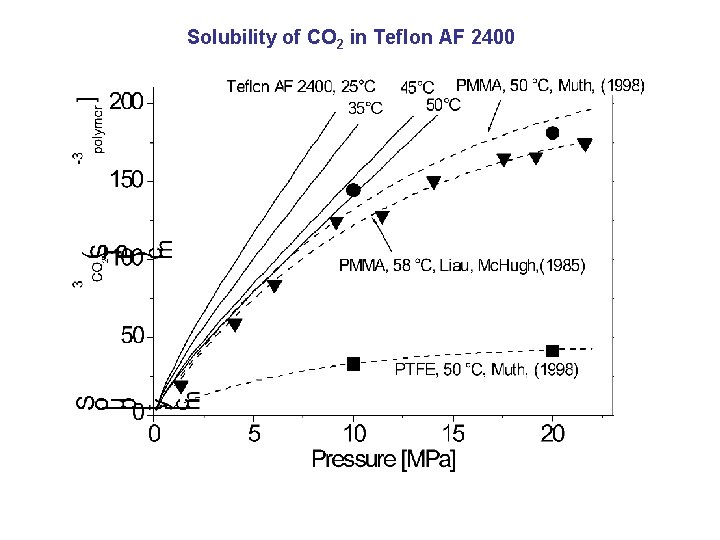
Solubility of CO 2 in Teflon AF 2400
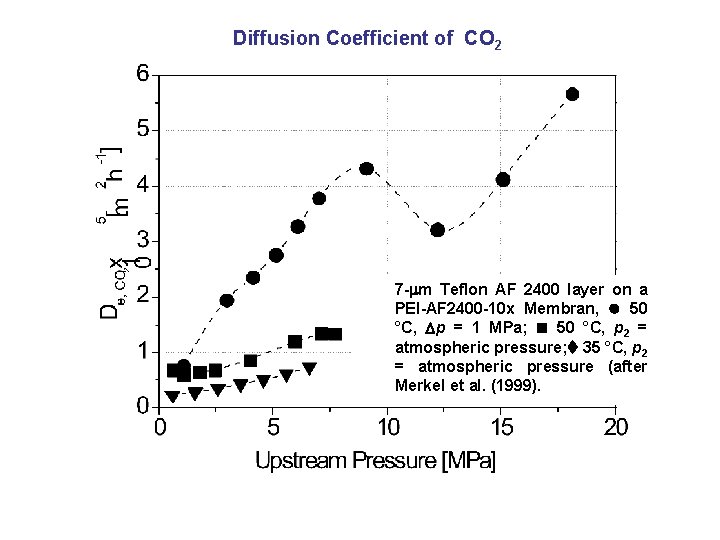
Diffusion Coefficient of CO 2 7 - m Teflon AF 2400 layer on a PEI-AF 2400 -10 x Membran, 50 °C, p = 1 MPa; 50 °C, p 2 = atmospheric pressure; 35 °C, p 2 = atmospheric pressure (after Merkel et al. (1999).
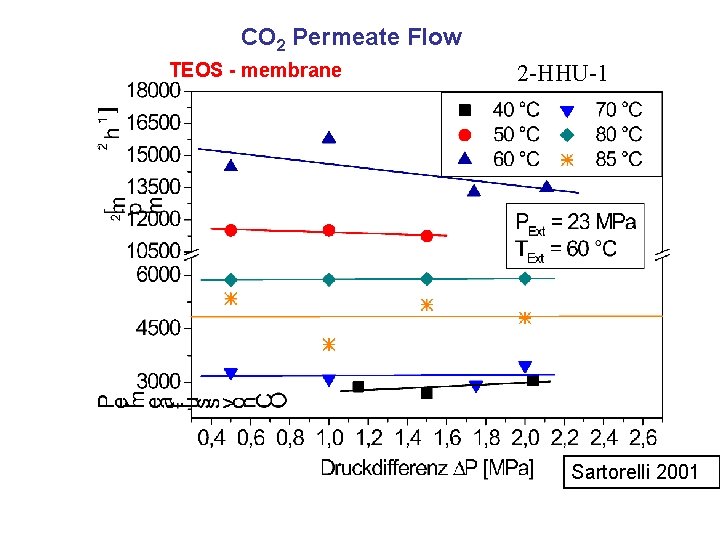
CO 2 Permeate Flow TEOS - membrane 2 -HHU-1 Sartorelli 2001
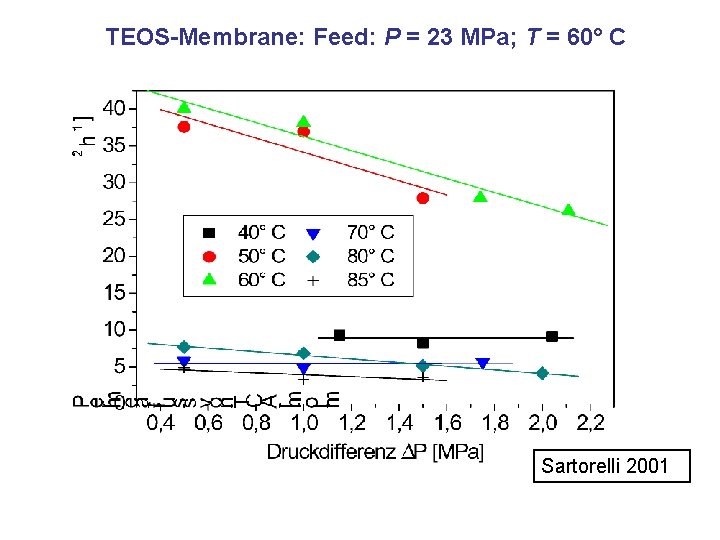
TEOS-Membrane: Feed: P = 23 MPa; T = 60° C Sartorelli 2001
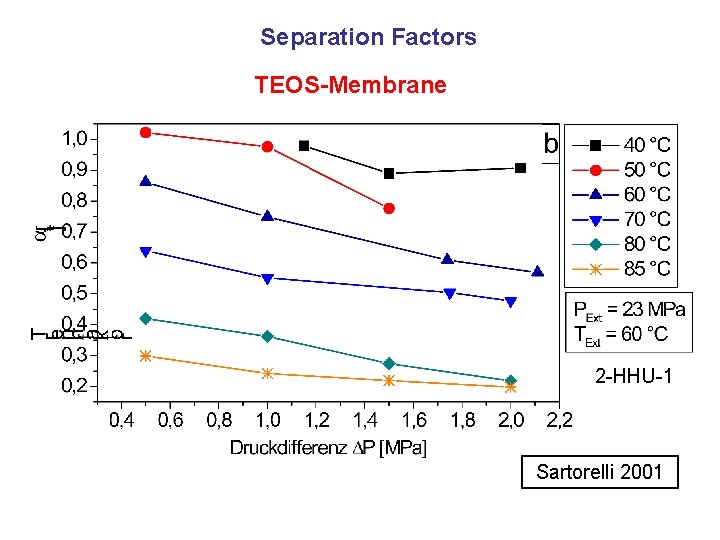
Separation Factors TEOS-Membrane 2 -HHU-1 Sartorelli 2001
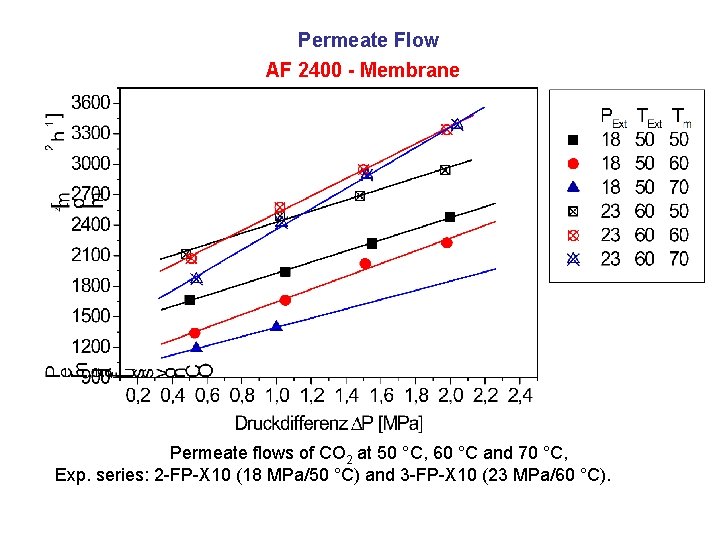
Permeate Flow AF 2400 - Membrane Permeate flows of CO 2 at 50 °C, 60 °C and 70 °C, Exp. series: 2 -FP-X 10 (18 MPa/50 °C) and 3 -FP-X 10 (23 MPa/60 °C).
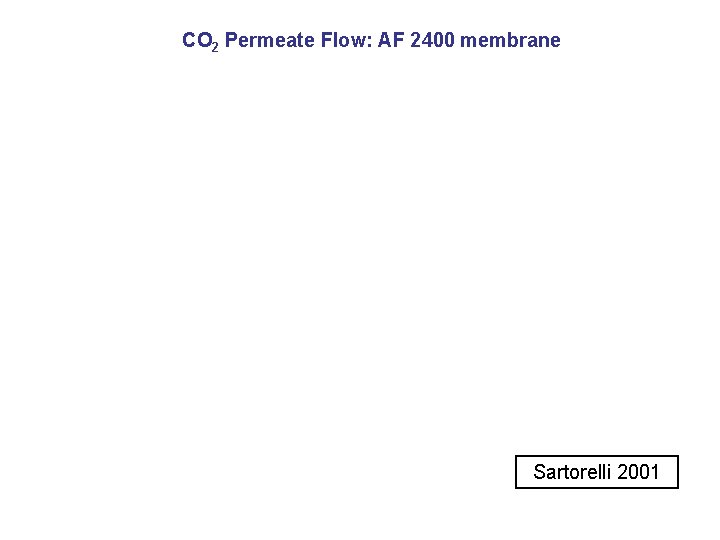
CO 2 Permeate Flow: AF 2400 membrane Sartorelli 2001
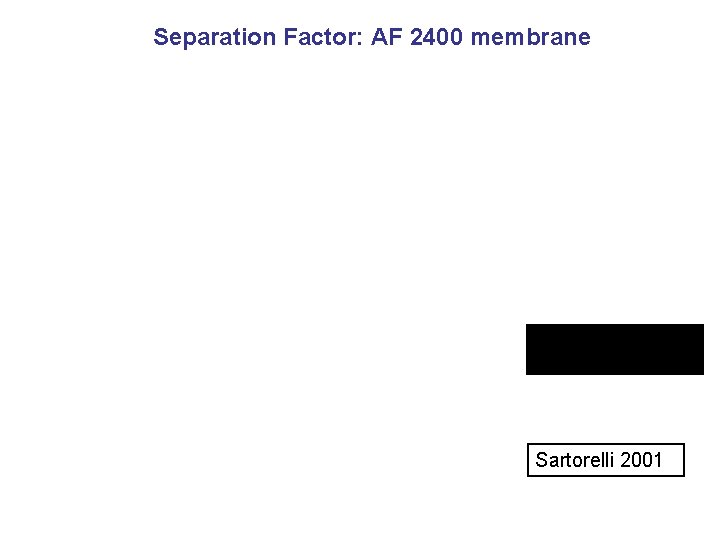
Separation Factor: AF 2400 membrane Sartorelli 2001
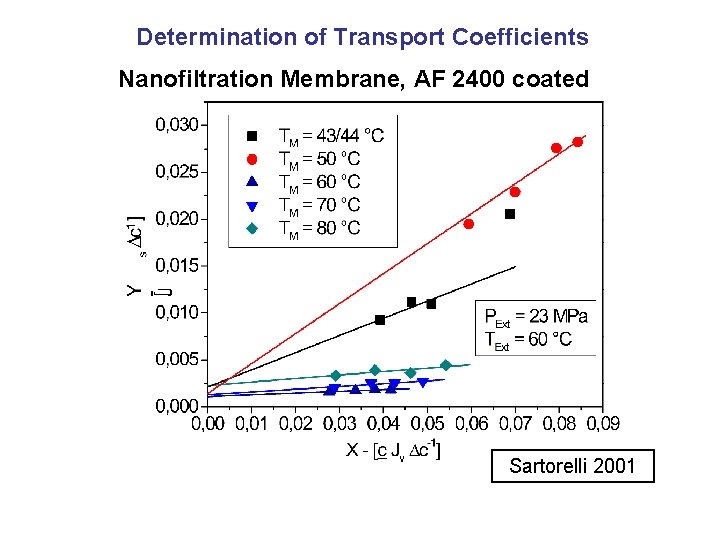
Determination of Transport Coefficients Nanofiltration Membrane, AF 2400 coated Sartorelli 2001
![Pressure Difference, PEI-FP, 323 3 -2 -1 J CO 2 [kmol m h ] Pressure Difference, PEI-FP, 323 3 -2 -1 J CO 2 [kmol m h ]](http://slidetodoc.com/presentation_image_h/b99b70034b9d4e6c4e1ba6f039a17ea5/image-52.jpg)
Pressure Difference, PEI-FP, 323 3 -2 -1 J CO 2 [kmol m h ] 6 5 4 3 7 MPa 9 MPa 12 MPa 14 MPa 16 MPa 18 MPa 2 1 0 0 1 2 3 4 p [MPa] 5 6
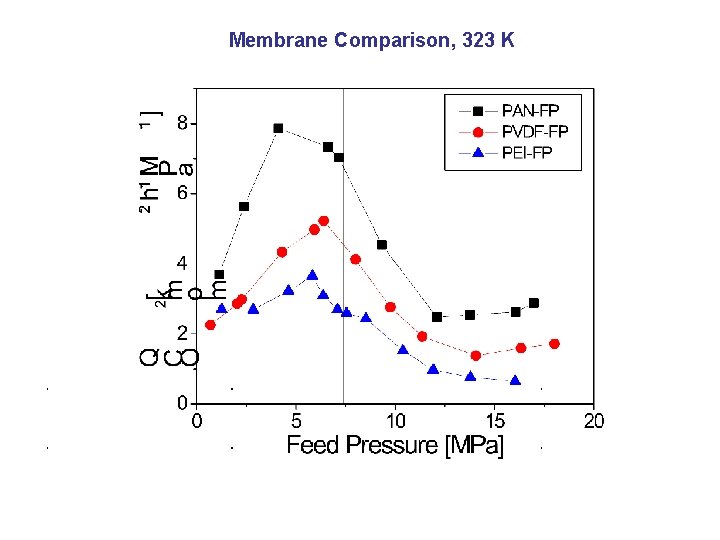
Membrane Comparison, 323 K
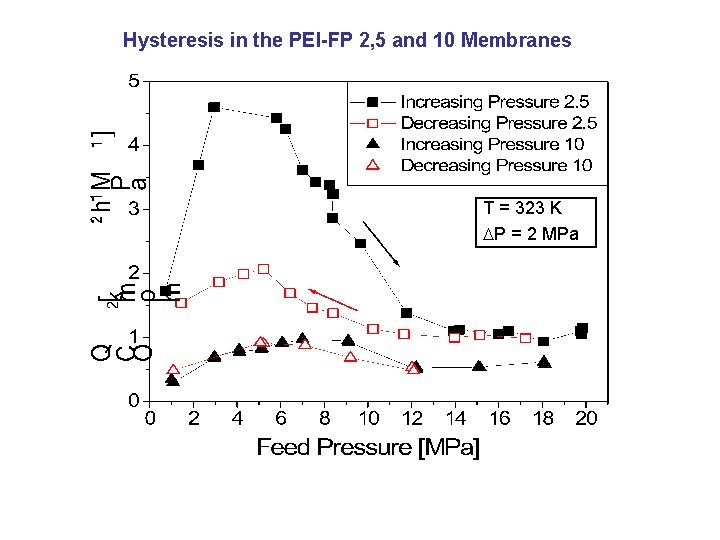
Hysteresis in the PEI-FP 2, 5 and 10 Membranes T = 323 K P = 2 MPa
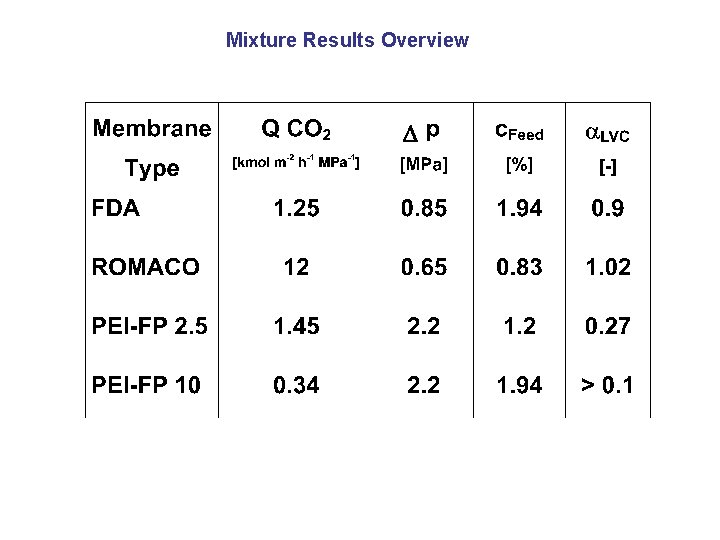
Mixture Results Overview
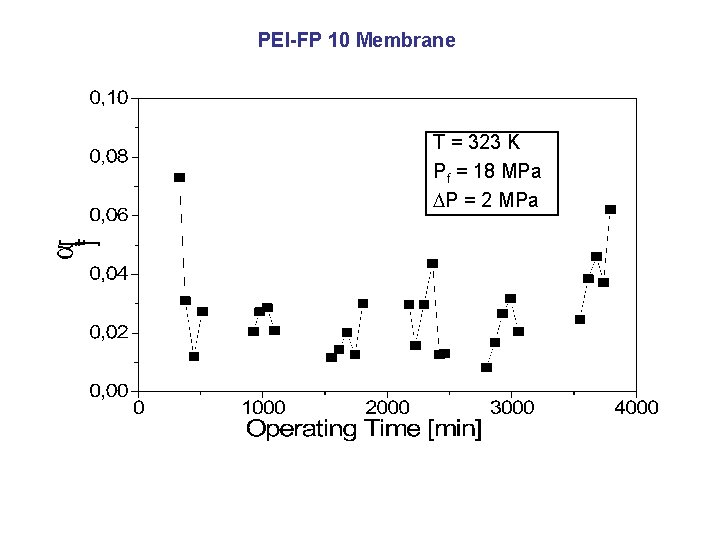
PEI-FP 10 Membrane T = 323 K Pf = 18 MPa P = 2 MPa
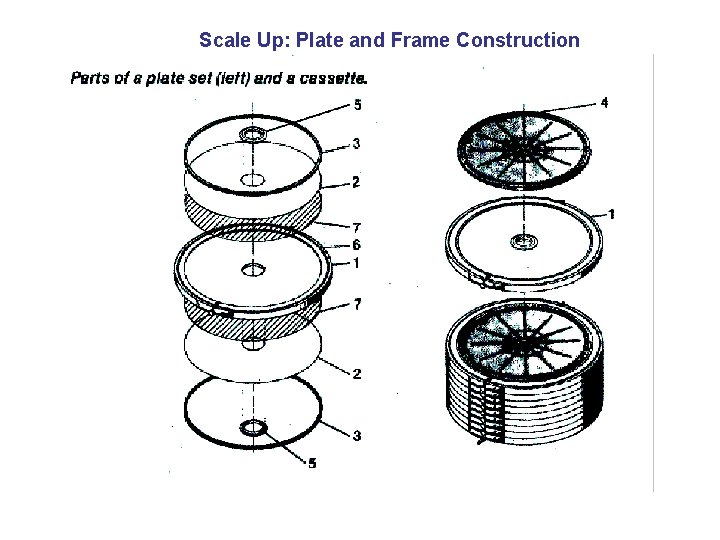
Scale Up: Plate and Frame Construction
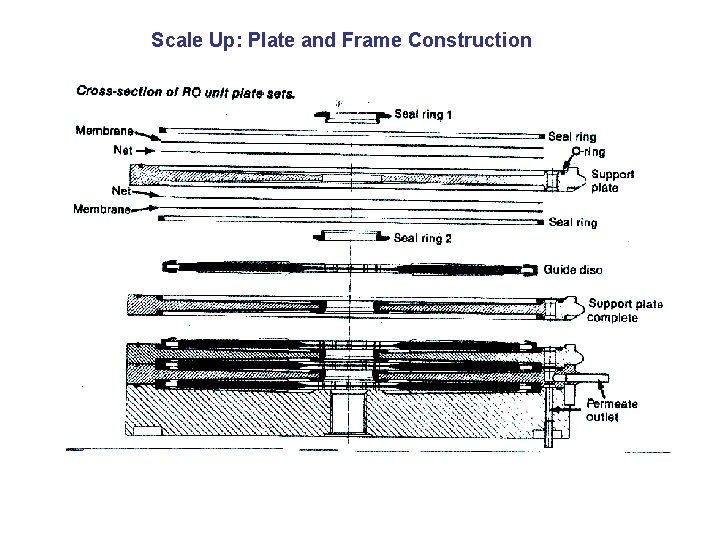
Scale Up: Plate and Frame Construction

Scale Up: Plate and Frame Construction
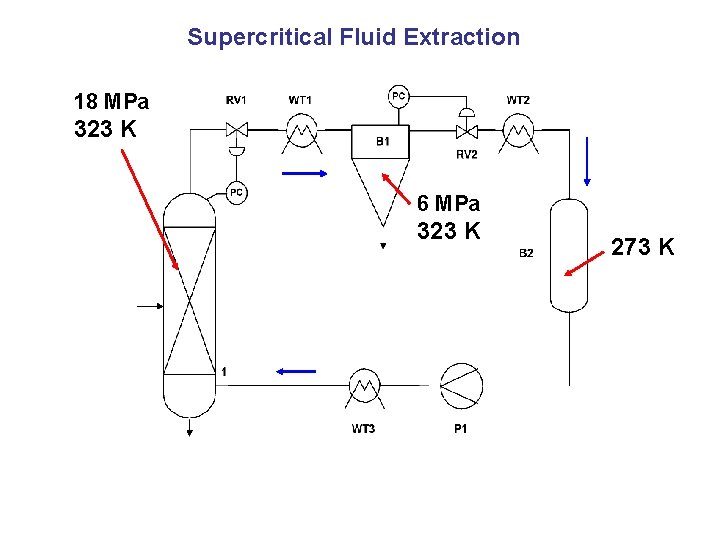
Supercritical Fluid Extraction 18 MPa 323 K 6 MPa 323 K 273 K
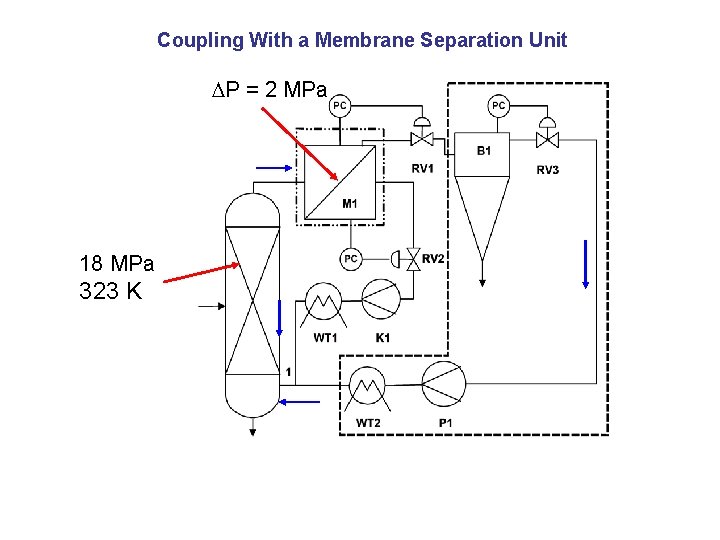
Coupling With a Membrane Separation Unit P = 2 MPa 18 MPa 323 K
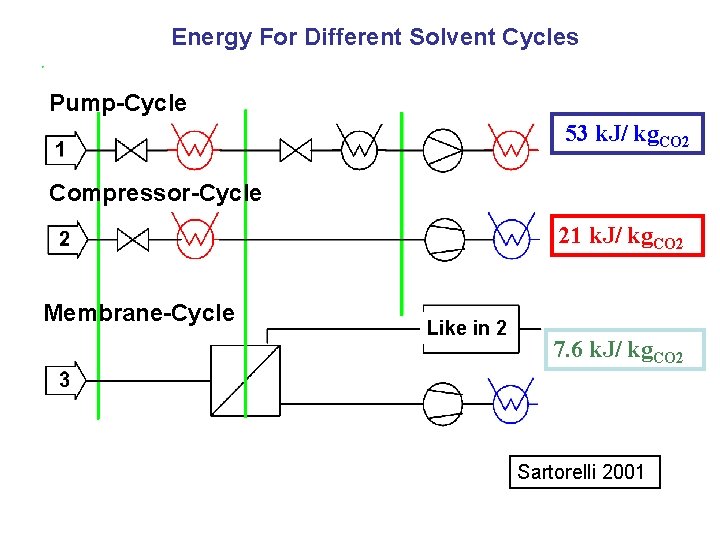
Energy For Different Solvent Cycles Pump-Cycle 53 k. J/ kg. CO 2 Compressor-Cycle 21 k. J/ kg. CO 2 Membrane-Cycle Like in 2 7. 6 k. J/ kg. CO 2 Sartorelli 2001
- Slides: 62