Ch 8 The Mole I Molar Conversions I







- Slides: 14

Ch. 8 – The Mole I. Molar Conversions I II IV

A. What is the Mole? n A counting number (like a dozen) n Also know as: Avogadro’s number n 1 mole = 6. 02 1023 items, that is: 602, 000, 000, 000 !!! A large amount!!!!

A. What is the Mole? n 1 mole of basketballs would fill a bag the size of the earth! n 1 mole of pennies would cover the Earth 1/4 mile deep! § If one mole of pennies were divided evenly among the 7 billion humans on earth, every person would have enough money to spend over 5 million dollars a day!

B. Molar Mass n Mass of 1 mole of an element or compound. n Atomic mass tells the. . . · atomic mass units per atom (amu) – based on Carbon-12 · grams per mole (g/mol) n Round to 2 decimal places (the hundredths place, always )

B. Molar Mass Examples n carbon 12. 01 g/mol n aluminum 26. 98 g/mol n zinc 65. 41 g/mol

B. Molar Mass Examples n water · H 2 O · 2(1. 01) + 16. 00 = 18. 02 g/mol n sodium chloride · Na. Cl · 22. 99 + 35. 45 = 58. 44 g/mol

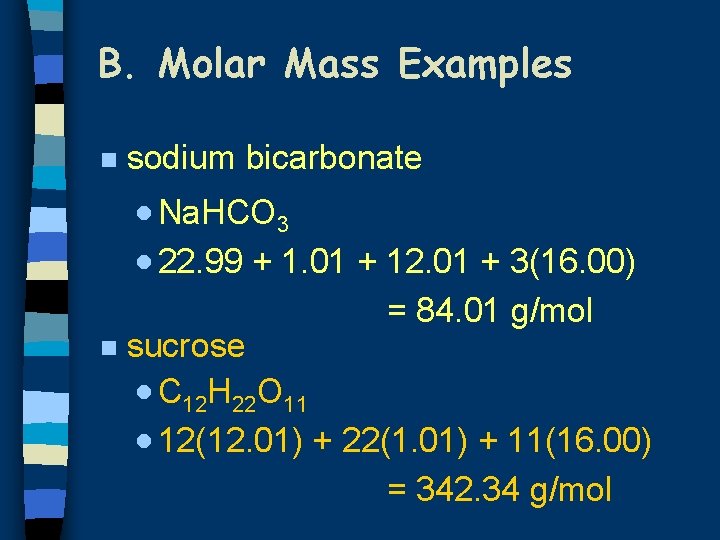
B. Molar Mass Examples n sodium bicarbonate · Na. HCO 3 · 22. 99 + 1. 01 + 12. 01 + 3(16. 00) = 84. 01 g/mol n sucrose · C 12 H 22 O 11 · 12(12. 01) + 22(1. 01) + 11(16. 00) = 342. 34 g/mol

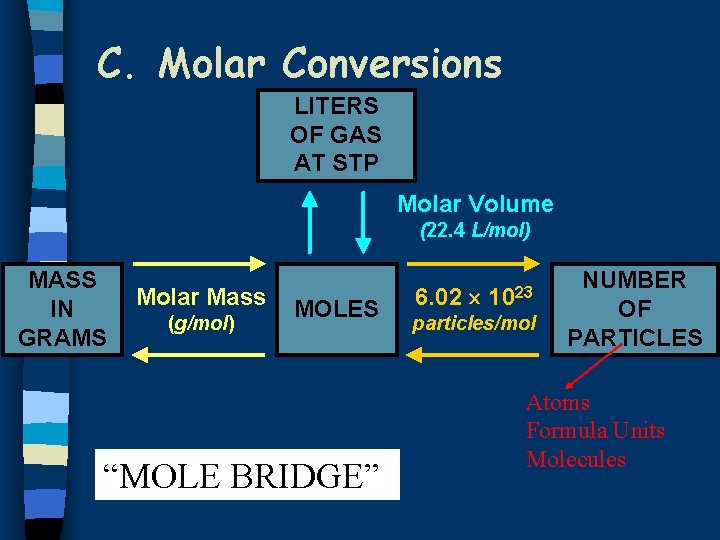
C. Molar Conversions LITERS OF GAS AT STP Molar Volume (22. 4 L/mol) MASS IN GRAMS Molar Mass (g/mol) MOLES “MOLE BRIDGE” 6. 02 1023 particles/mol NUMBER OF PARTICLES Atoms Formula Units Molecules

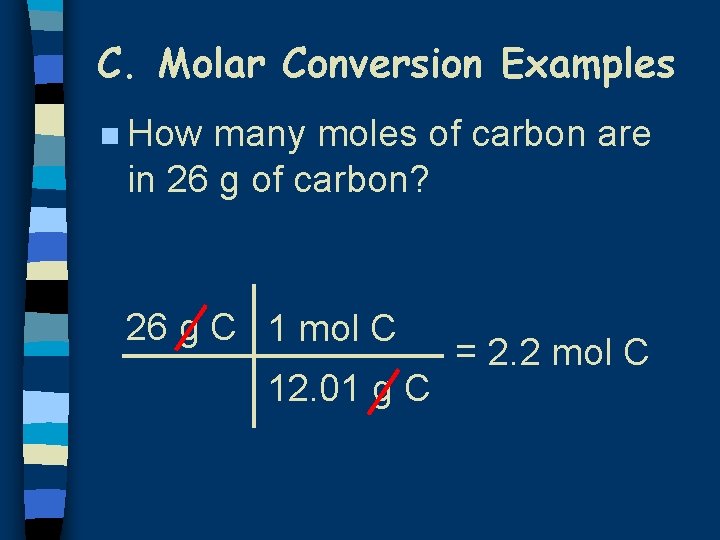
C. Molar Conversion Examples n How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C 12. 01 g C = 2. 2 mol C

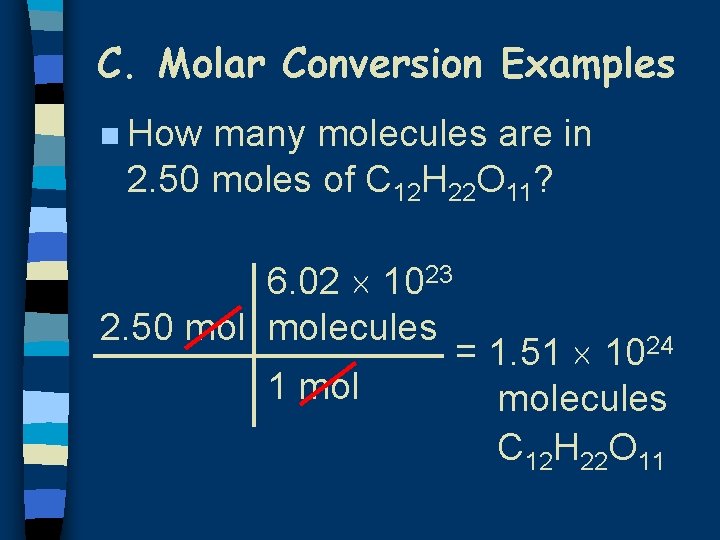
C. Molar Conversion Examples n How many molecules are in 2. 50 moles of C 12 H 22 O 11? 6. 02 1023 2. 50 molecules 1 mol = 1. 51 1024 molecules C 12 H 22 O 11

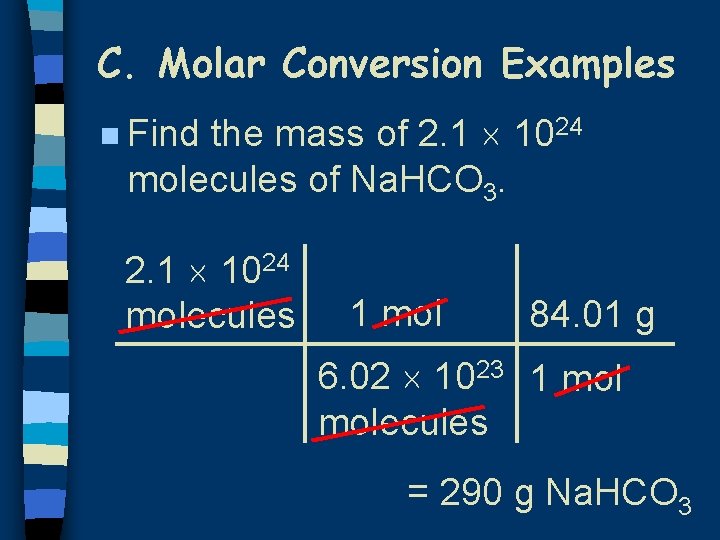
C. Molar Conversion Examples the mass of 2. 1 1024 molecules of Na. HCO 3. n Find 2. 1 1024 molecules 1 mol 84. 01 g 6. 02 1023 1 molecules = 290 g Na. HCO 3

D. Molar Volume Avogadro proposed at the same temperature and pressure, equal volumes of gases contain the same number of particles n 1 mole of gas at STP (Oo. C, 1 atm) has a volume of 22. 4 liters n STP = standard temperature & pressure n

D. Molar Volume n A reaction produces 0. 82 moles O 2. What volume is this at STP? 0. 82 mol 22. 4 L 1 mole = 18 L O 2

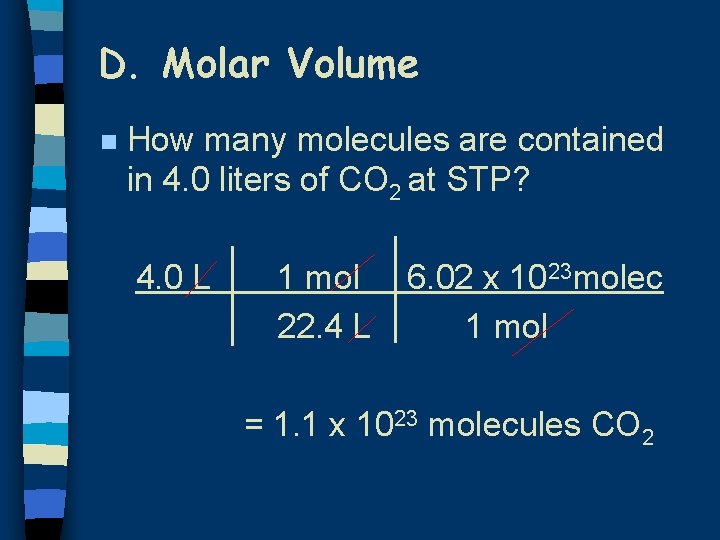
D. Molar Volume n How many molecules are contained in 4. 0 liters of CO 2 at STP? 4. 0 L 1 mol 22. 4 L 6. 02 x 1023 molec 1 mol = 1. 1 x 1023 molecules CO 2