Ch 8 1 Properties of Solids Physical properties































- Slides: 55

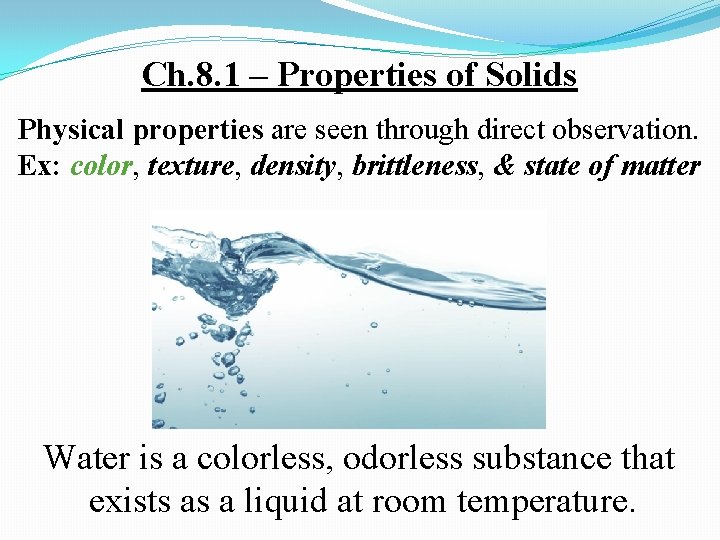
Ch. 8. 1 – Properties of Solids Physical properties are seen through direct observation. Ex: color, texture, density, brittleness, & state of matter Water is a colorless, odorless substance that exists as a liquid at room temperature.

Physical change: A change in size, shape, or phase of matter that doesn’t change a substance’s identity. Ex: When water is frozen, it’s still water and the change can be easily reversed by melting.

Chemical properties can only be observed when one substance changes into a different substance. Ex: If you leave an iron nail outside, the iron reacts with oxygen in the air to form iron oxide (rust).

Chemical changes are not easily reversible. Ex: Rusted iron will not turn shiny again, even if you take it away from oxygen in the air.

Density is the ratio of mass to volume: Homogeneous materials are the same density throughout. Ex: a steel nail & a steel cube have the same density, even though they are different sizes.

Platinum is one of the densest metals, with a density of 21, 500 kg/m 3! It’s twice as dense as lead & ~ 3 times as dense as steel!

A material’s density depends on 2 things: 1. The individual masses of the atoms or molecules. (MASS) 2. How closely they are packed together. (VOLUME)

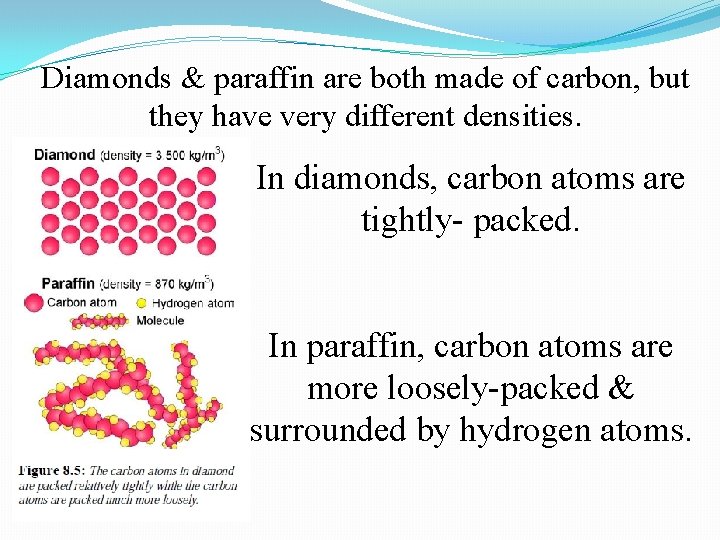
Diamonds & paraffin are both made of carbon, but they have very different densities. In diamonds, carbon atoms are tightly- packed. In paraffin, carbon atoms are more loosely-packed & surrounded by hydrogen atoms.

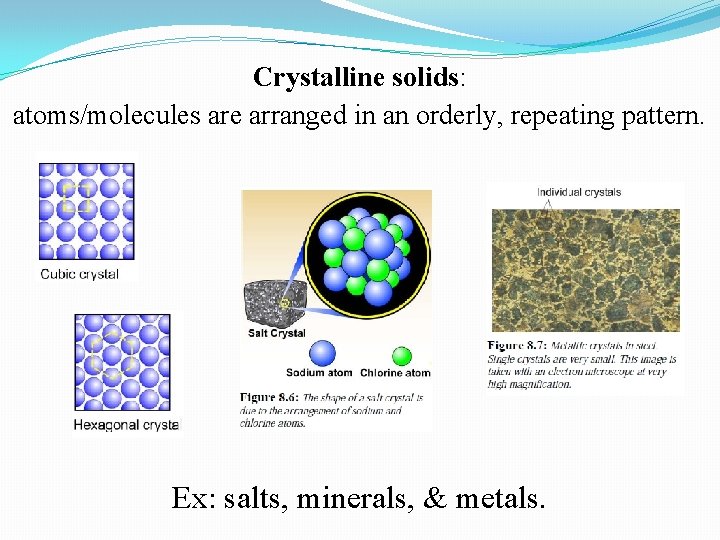
Crystalline solids: atoms/molecules are arranged in an orderly, repeating pattern. Ex: salts, minerals, & metals.

Amorphous solids: -atoms/molecules are arranged in a random way - hold their shape - are softer & more elastic than crystalline solids. Ex: rubber, wax, & glass.

An object’s strength depends on 2 things: - How much it bends or deforms when force is applied - How much force it can endure before it breaks • Strength comes from design & materials.

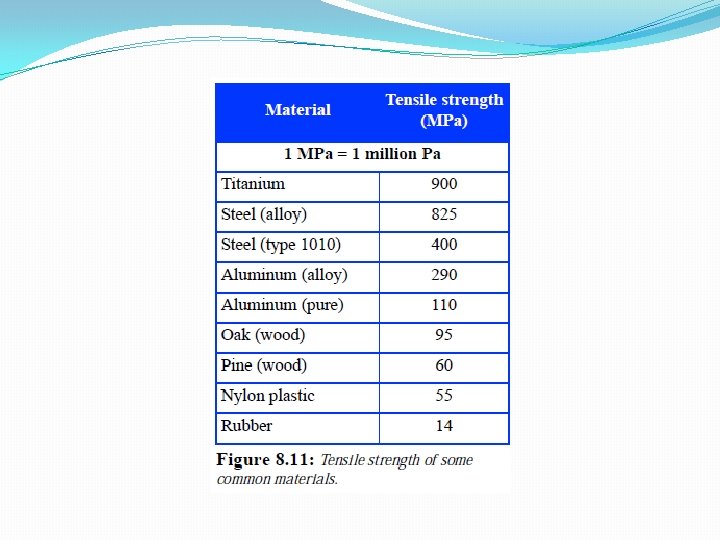
- The metric unit is the pascal (Pa). 1 pascal = 1 N/m 2 - The English unit is pounds per square inch (psi). 1 psi = 1 lb/in 2 • Strong materials like steel & aluminum can take stresses of 1 million pascals!

Tensile strength: the tension a material can withstand before breaking. • Strong materials like steel have high tensile strength. • Weak materials have low tensile strength. (wax, rubber, glass)


Elasticity: ability to be stretched & return to original size Elastic objects have the ability to bounce & withstand impact without breaking.

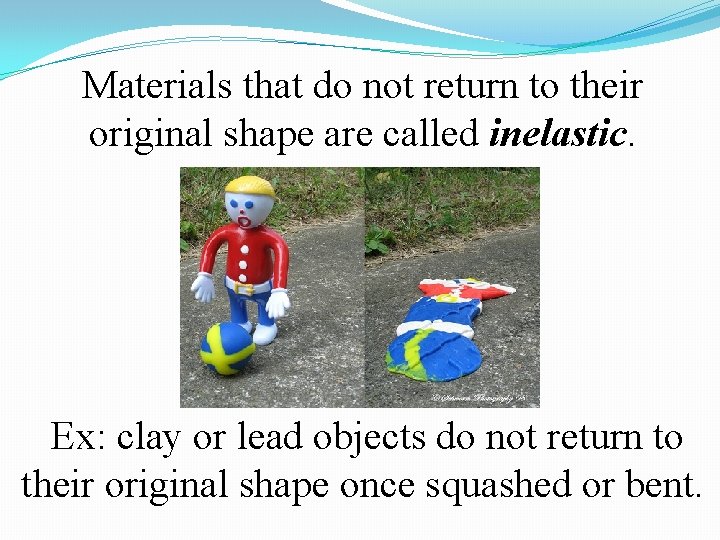
Materials that do not return to their original shape are called inelastic. Ex: clay or lead objects do not return to their original shape once squashed or bent.

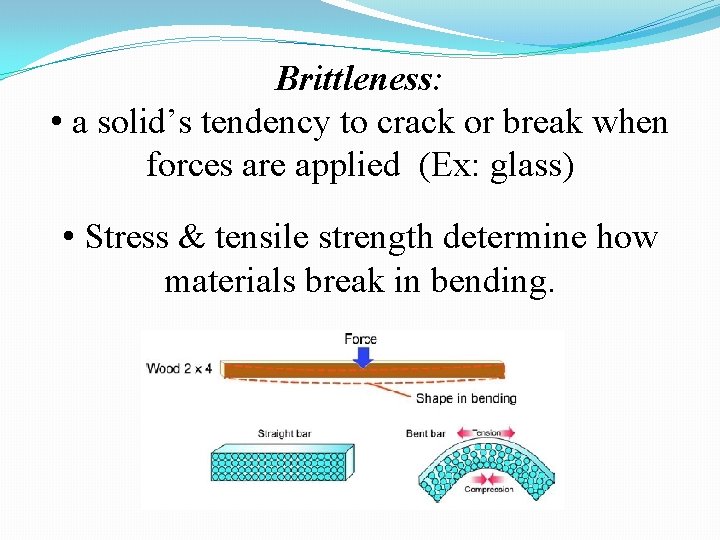
Brittleness: • a solid’s tendency to crack or break when forces are applied (Ex: glass) • Stress & tensile strength determine how materials break in bending.

• As temperature increases, kinetic energy in the atoms & molecules also increases. • The increased vibration makes each particle take up a little more space, causing thermal expansion. • Almost all solid materials expand as temperature increases. • Some materials expand a great deal (plastic), while others expand only a little (glass).

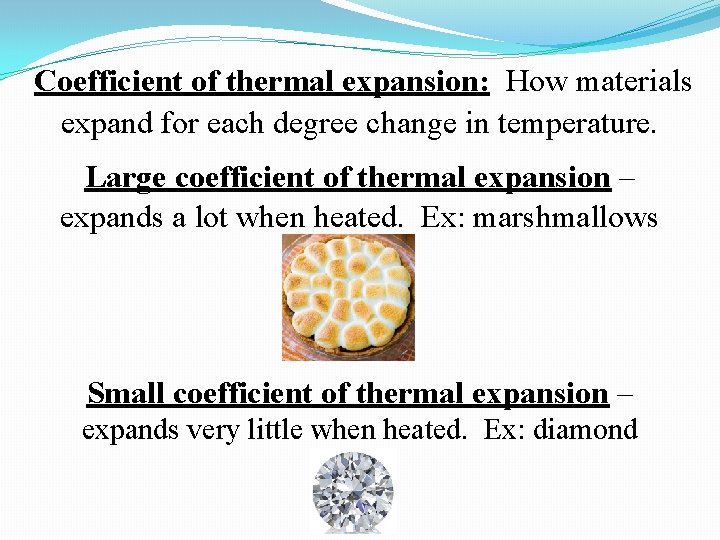
Coefficient of thermal expansion: How materials expand for each degree change in temperature. Large coefficient of thermal expansion – expands a lot when heated. Ex: marshmallows Small coefficient of thermal expansion – expands very little when heated. Ex: diamond

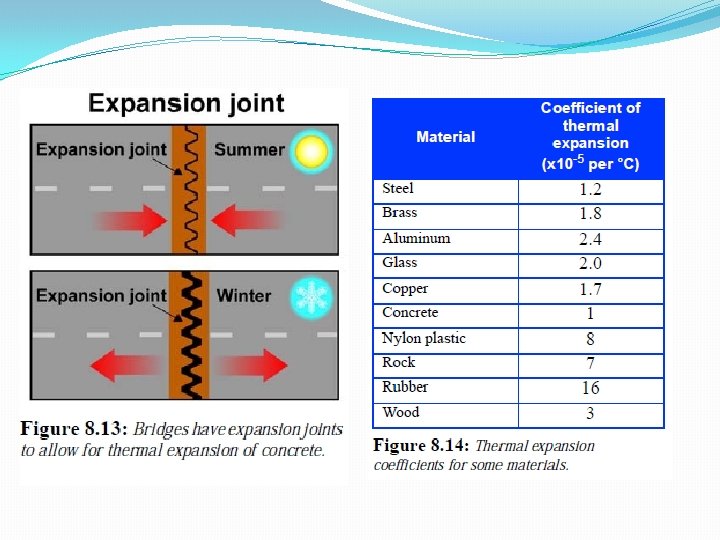
• If temperature decreases, objects contract. • Large stresses can develop if objects are prevented from expanding or contracting with temperature changes. • Bridges & roads have expansion joints to allow for temperature fluctuations.

Bridge joints are designed to expand & contract. They absorb vibration & allow movement in case of an earthquake.


Ch. 8. 2 – Fluids Fluid: matter that flows when force is applied. Ex: liquids & gases.

Recall: • Density changes with phase changes Ex: If you melt a silver candlestick, its mass doesn’t change, but its volume increases. (Liquid silver is less dense than solid silver)

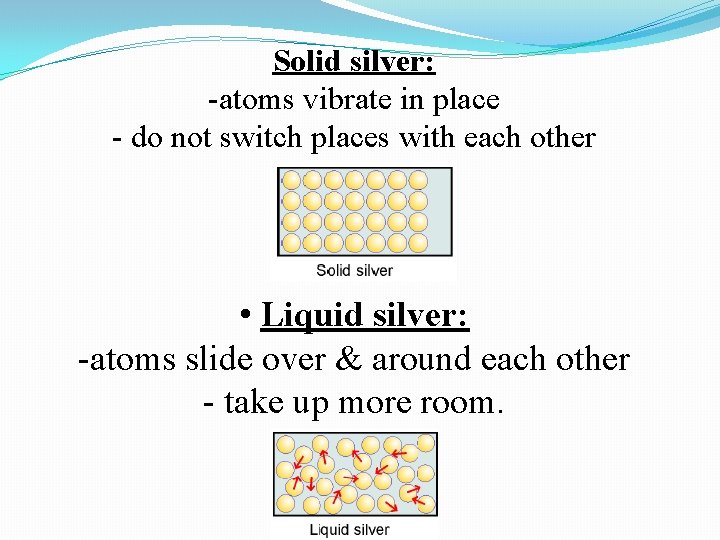
Solid silver: -atoms vibrate in place - do not switch places with each other • Liquid silver: -atoms slide over & around each other - take up more room.

Why are liquids less dense than solids? A solid’s atoms are tightly packed & organized. A liquid’s atoms still touch, but no longer fit in the same volume.

Most materials are more dense in their solid state than in their liquid state. One notable exception is water! Density of ice = 920 kg/m 3 Density of water = 1, 000 kg/m 3

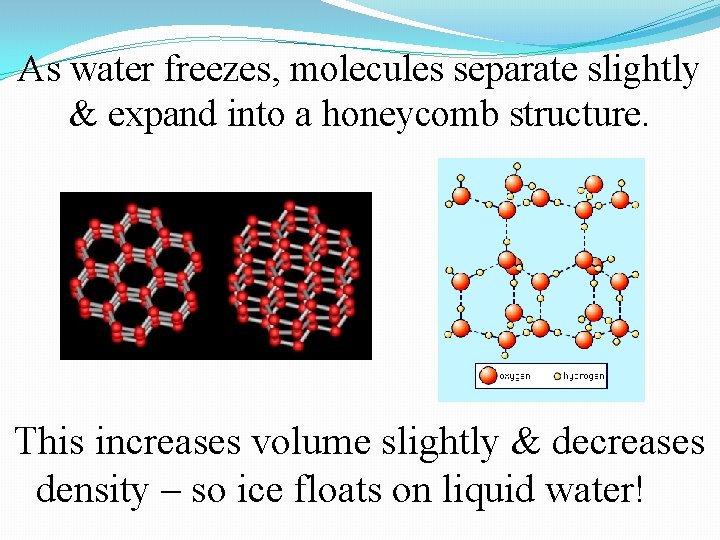
Solid water has an open crystal structure that resembles a honeycomb. This 6 -sided crystal form explains the shape of snowflakes.

As water freezes, molecules separate slightly & expand into a honeycomb structure. This increases volume slightly & decreases density – so ice floats on liquid water!

Ice is less dense than water. It floats on the surface of frozen lakes & ponds in the winter. The temperature of the water under the ice stays above freezing, so fish & other aquatic life survive.

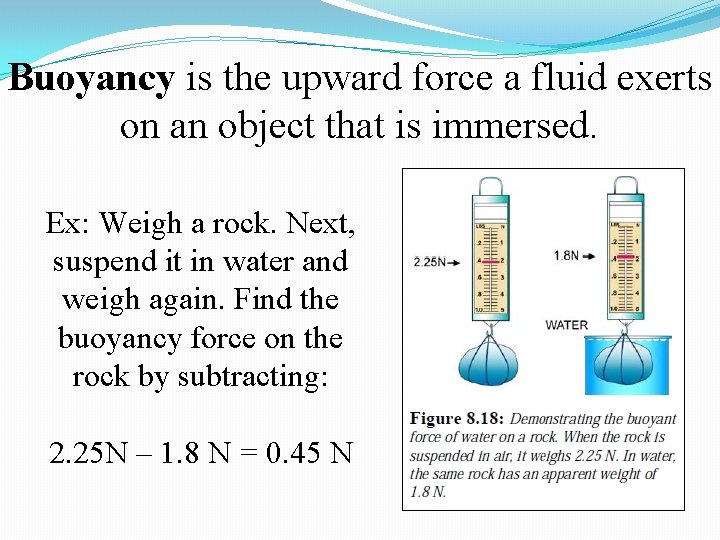
Buoyancy is the upward force a fluid exerts on an object that is immersed. Ex: Weigh a rock. Next, suspend it in water and weigh again. Find the buoyancy force on the rock by subtracting: 2. 25 N – 1. 8 N = 0. 45 N

Free-body diagram: buoyant force & weight

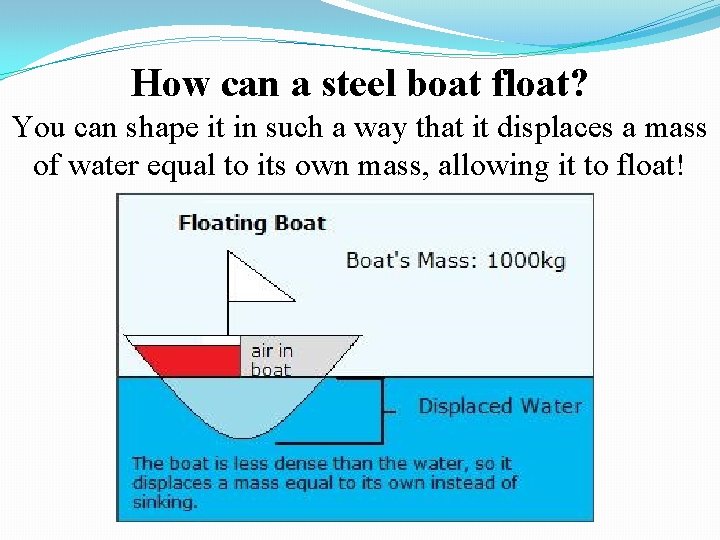
How can a steel boat float? You can shape it in such a way that it displaces a mass of water equal to its own mass, allowing it to float!

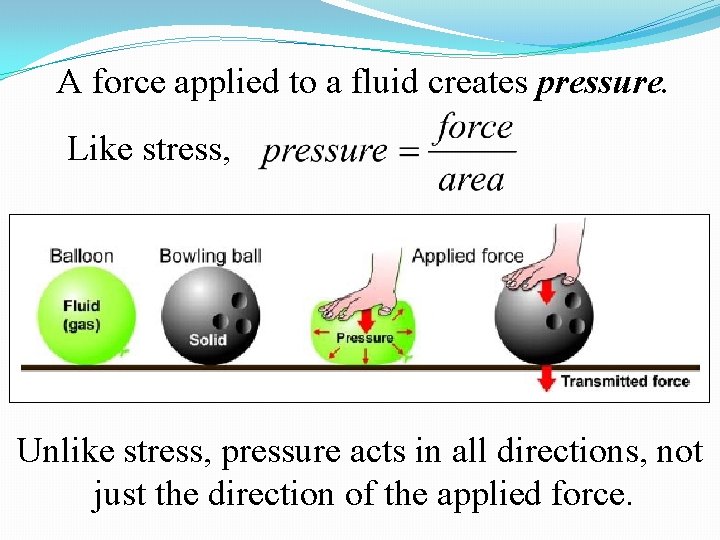
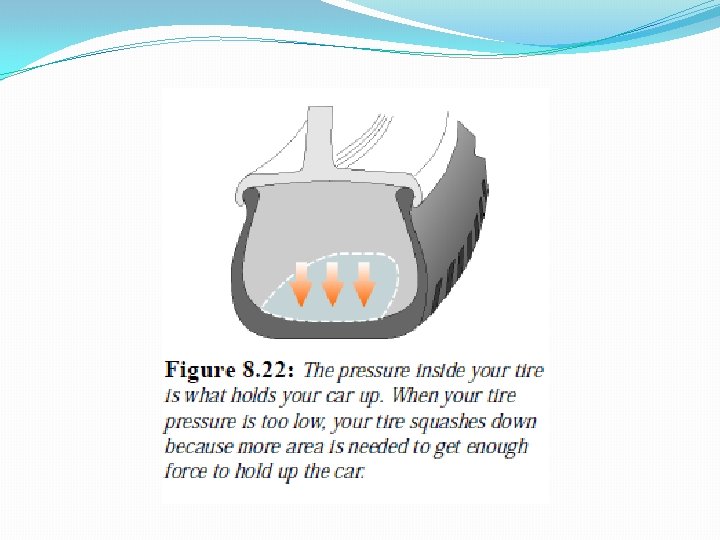
A force applied to a fluid creates pressure. Like stress, Unlike stress, pressure acts in all directions, not just the direction of the applied force.

Gravity creates pressure because fluids have weight.

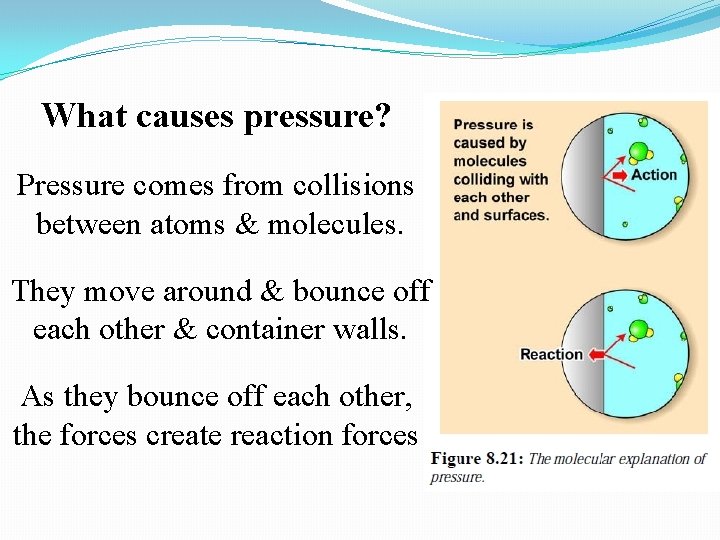
What causes pressure? Pressure comes from collisions between atoms & molecules. They move around & bounce off each other & container walls. As they bounce off each other, the forces create reaction forces


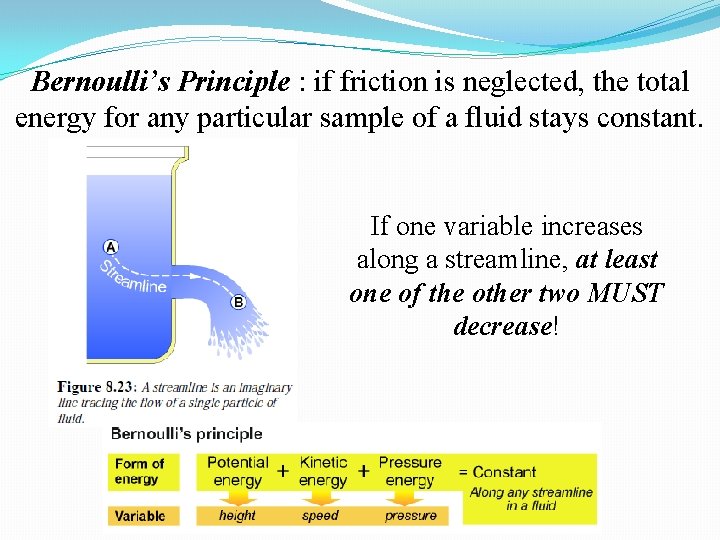
Bernoulli’s Principle : if friction is neglected, the total energy for any particular sample of a fluid stays constant. If one variable increases along a streamline, at least one of the other two MUST decrease!

The shape of an airfoil causes air flowing along the top (A) to move faster than air flowing along the bottom (B). According to Bernoulli’s Principle, if the speed goes up, the pressure goes down. The difference in pressure is what creates the lift force that supports the plane in the air.

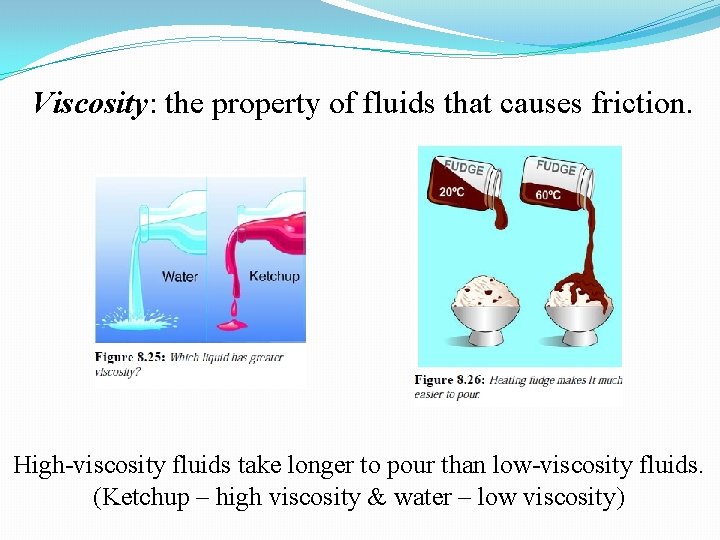
Viscosity: the property of fluids that causes friction. High-viscosity fluids take longer to pour than low-viscosity fluids. (Ketchup – high viscosity & water – low viscosity)

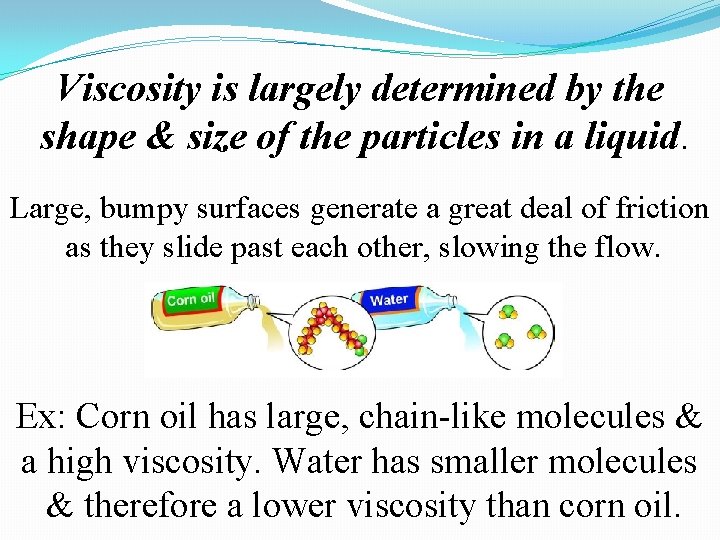
Viscosity is largely determined by the shape & size of the particles in a liquid. Large, bumpy surfaces generate a great deal of friction as they slide past each other, slowing the flow. Ex: Corn oil has large, chain-like molecules & a high viscosity. Water has smaller molecules & therefore a lower viscosity than corn oil.

Raising a liquid’s temperature decreases its viscosity. Warmer liquids are easier to pour than cooler liquids – raising the temperature increases the jiggling of the molecules, making it easier for them to slide past one another. As a result, the viscosity decreases.

Ch. 8. 3 – The Behavior of Gases • While gases are fluids, they are different than liquids because the molecules in a gas are completely separated from each other. • Because gas molecules act independently, gases are free to expand or contract. • A gas will expand to completely fill its container.

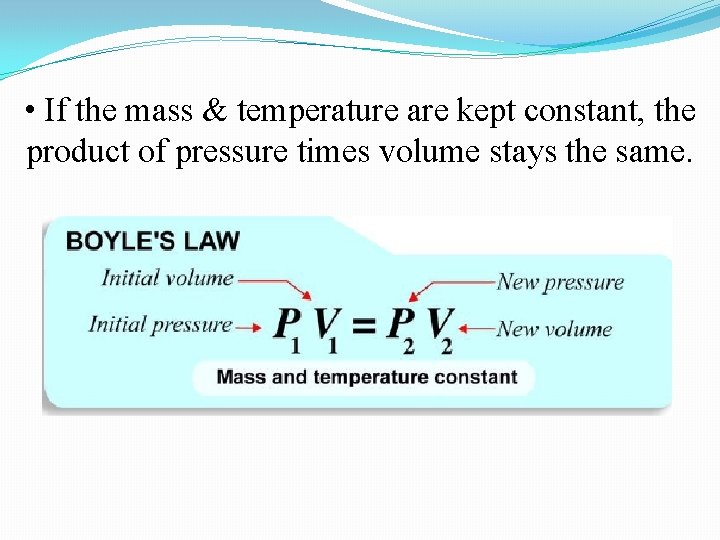
• When you squeeze a fixed quantity of gas into a fixed volume, the pressure goes up. This rule is known as Boyle’s Law. • The pressure increases because the same number of molecules are squeezed into a smaller space. • The molecules hit the walls more often because there are more of them per unit of area.

• If the mass & temperature are kept constant, the product of pressure times volume stays the same.

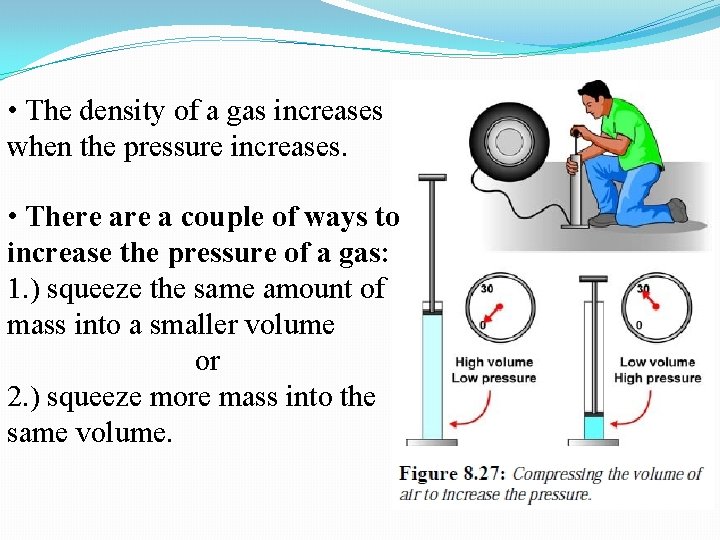
• The density of a gas increases when the pressure increases. • There a couple of ways to increase the pressure of a gas: 1. ) squeeze the same amount of mass into a smaller volume or 2. ) squeeze more mass into the same volume.

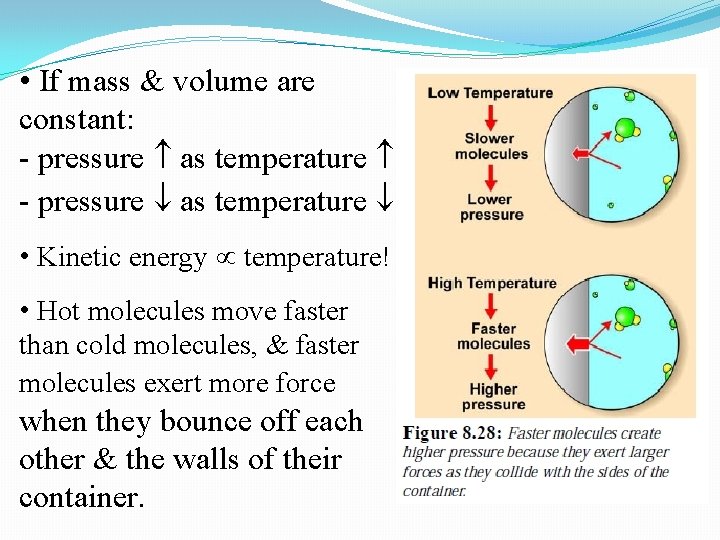
• If mass & volume are constant: - pressure as temperature • Kinetic energy temperature! • Hot molecules move faster than cold molecules, & faster molecules exert more force when they bounce off each other & the walls of their container.

• Like water, gases can create buoyancy forces. • Objects of lower density can float on gas of higher density. • A hot-air balloon floats because it is less dense than the surrounding air. • The heated air inside the balloon expands and becomes less dense than the cooler air around the balloon.

According to Charles’ Law: - the volume of a gas as temperature

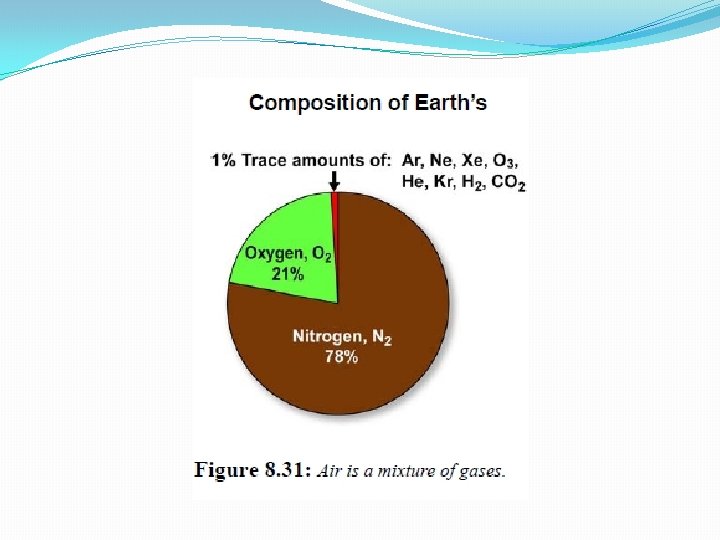
• Air feels “light” because it is 1, 000 times less dense than water. • Molecules of nitrogen(N 2) & oxygen(O 2) account for 97. 2 % of the mass of air. • The amount of water vapor in the air depends on temperature & relative humidity.


• Water vapor is water in gas form. • How does water vapor enter the air? - boiling water - evaporation • How does water vapor leave the air? - condensation

• Relative humidity tells how much water vapor is in the air compared to how much the air can hold. • When the relative humidity is 100%, the air has as much water as it can hold, so any water vapor that evaporates from your skin is condensed right back again. • This is why you feel hot and sticky when humidity is high.

• In dry climates, however, the opposite is true. • Hot dry desert air has a very low relative humidity, allowing water to evaporate very quickly. • This is why dry heat feels more bearable than humid heat.

The End