Ch 7 Review Ionic Compounds True or False













- Slides: 19

Ch 7 Review Ionic Compounds

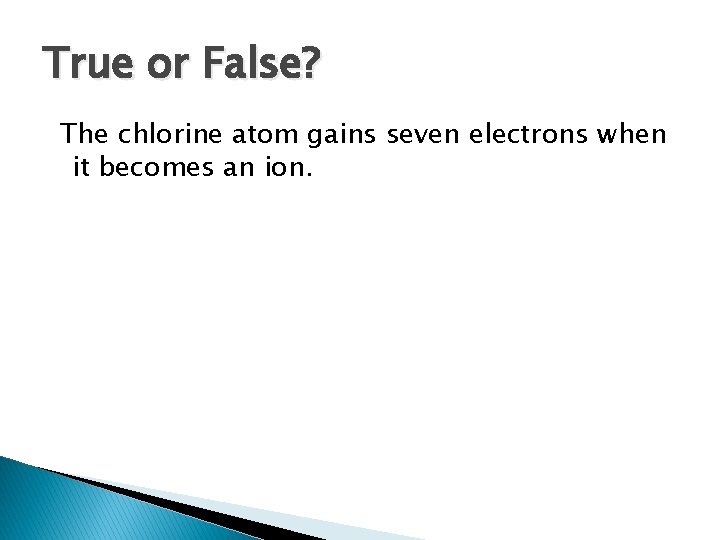
True or False? The chlorine atom gains seven electrons when it becomes an ion.

What is the formula for sodium chloride? A) B) C) D) Na. Cl Cl. Na Na 7 Cl 1 SCl

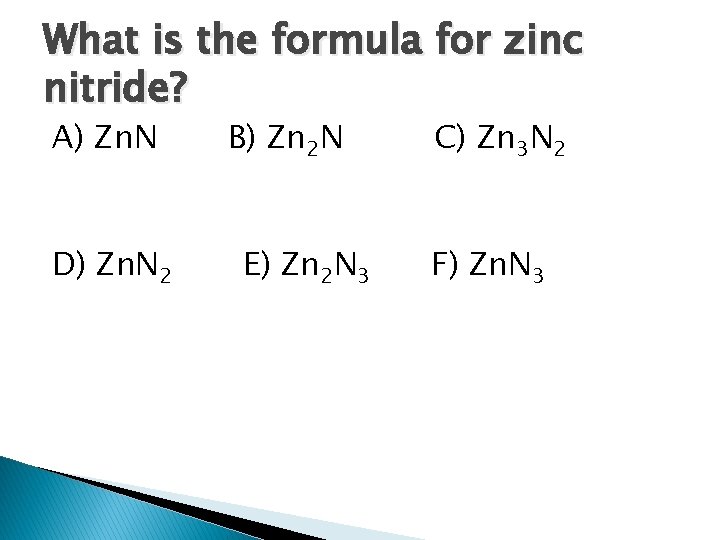
What is the formula for zinc nitride? A) Zn. N D) Zn. N 2 B) Zn 2 N E) Zn 2 N 3 C) Zn 3 N 2 F) Zn. N 3

What is the name given to the electrons in the highest occupied energy level? A) B) C) D) Anions Cations Valence electrons Orbital electrons

What is the typical charge of group 2? A) B) C) D) Negative two Positive two IDK Positive one

How many valence electrons does oxygen have? A) B) C) D) None One Six eight

True or False A cation is positively charged because it will gain electrons to have a stable electron configuration like a noble gas.

True or False The spirit colors at SEHS are red, white, and blue.

Yes or No �The charge of an anion is negative.

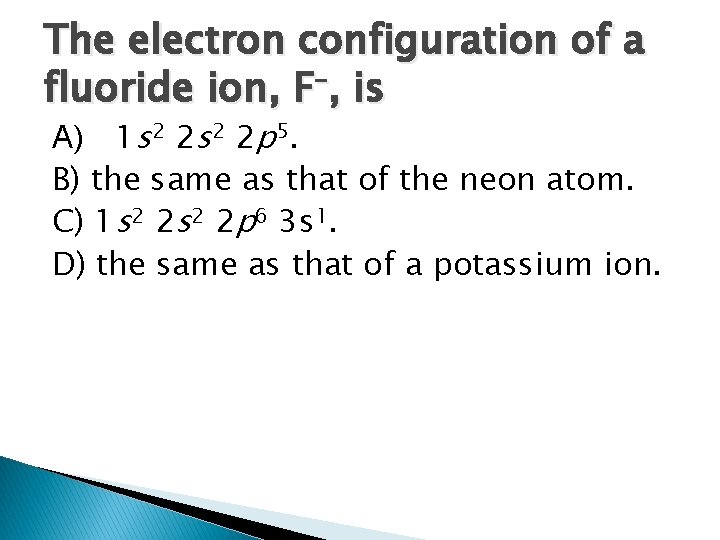
The electron configuration of a fluoride ion, F–, is A) 1 s 2 2 p 5. B) the same as that of the neon atom. C) 1 s 2 2 p 6 3 s 1. D) the same as that of a potassium ion.

Metals are good conductors of electricity because they a. form crystal lattices. b. contain positive ions. c. contain mobile valence electrons. d. form ionic bonds.

When an aluminum atom loses its valence electrons, what is the charge on the resulting ion? a. 2+ b. 2– c. 3+ d. 1+

The correct name for the N 3– ion is the: A) nitrate ion. B) nitric ion. C) nitride ion. D) nitrite ion.

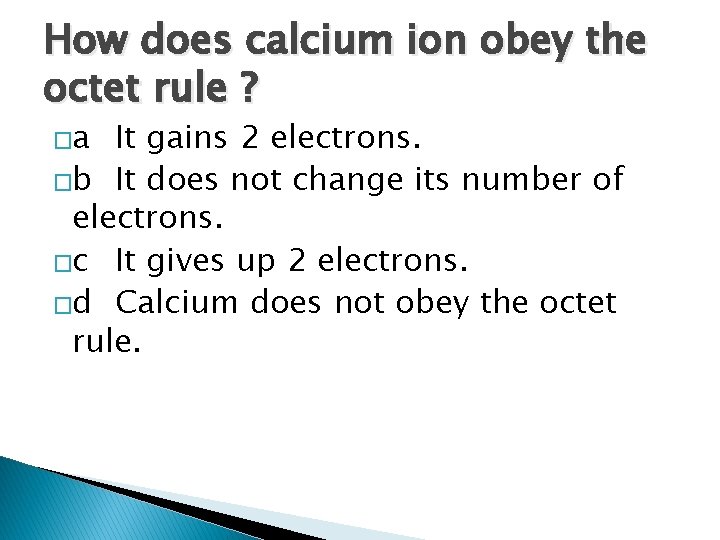
How does calcium ion obey the octet rule ? �a It gains 2 electrons. �b It does not change its number of electrons. �c It gives up 2 electrons. �d Calcium does not obey the octet rule.

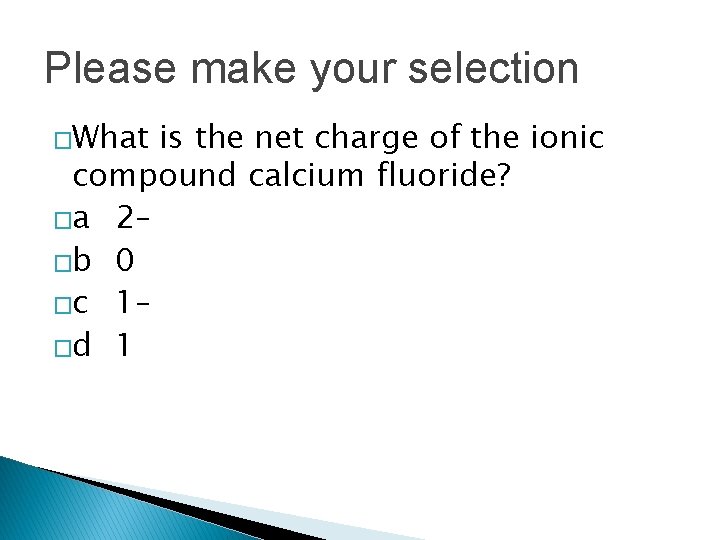
Please make your selection �What is the net charge of the ionic compound calcium fluoride? �a 2– �b 0 �c 1– �d 1

Please make your selection �What is the formula unit of sodium nitride? �a Na 3 N �b Na. N 3 �c Na 2 N �d Na. N 2

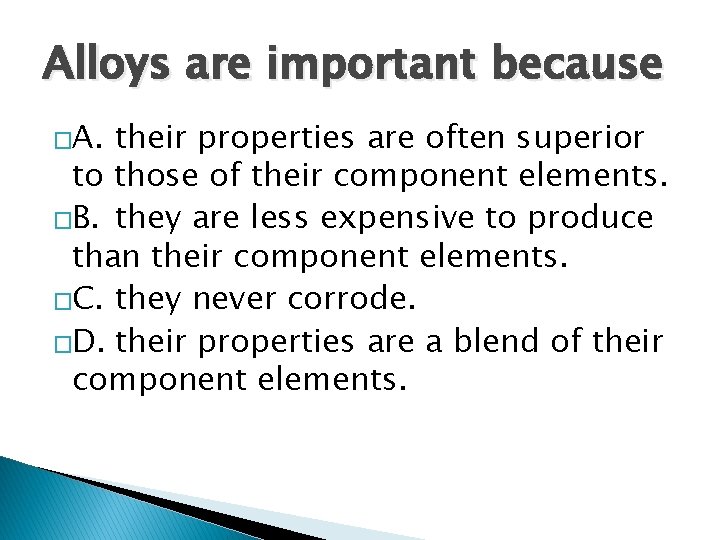
Alloys are important because �A. their properties are often superior to those of their component elements. �B. they are less expensive to produce than their component elements. �C. they never corrode. �D. their properties are a blend of their component elements.

Please make your selection �What characteristic of metals makes them good electrical conductors? �A. They have mobile cations. �B. They have mobile valence electrons �C. They have mobile protons. �D. Their crystal structures can be rearranged easily.