Ch 7 Ionic Bonds Chemical Bonds Chemical bonds

Ch. 7 Ionic Bonds

Chemical Bonds • Chemical bonds form when atoms are strongly attracted to one another 1) Ionic Bond 2) Covalent Bond 3) Metallic Bond

Compounds - Review • Compound: Substance that is composed of two or more elements that are combined chemically • Properties of a compound are generally very different from the elements that make it • Chemical Formulas: • Formulas show the symbols on the ration of the elements in the compound • Subscripts: tell the number of each element in the compound Ex. C 12 H 22 O 11

Review: • Valence Electrons: Electrons in the highest energy level • The number of valence electrons largely determines the chemical properties of an element. • To find the number of valence electrons, look at its group number. • Electron Dot Notation: represents the number of valence electrons

Ions - Review • Ion: an atom or bonded group of atoms that has a positive or negative charge • When atoms lose electrons and become positive ions, they always become smaller (compared to the neutral atom) • Loss of valence electron can leave an empty outer orbital resulting in a small radius • When atoms gain electrons and become negative ions, they become larger

Octet Rule • Atoms tend to gain, lose (ionic bond) or share (covalent bond) electrons until they are surrounded by eight valence electrons • An octet consists of 8 valence electrons • Since the noble gases have eight electrons, we assume that an atom is stable when surrounded by 8 valence electrons

A gain of one electron gives chlorine an octet and converts a chlorine atom into a chloride ion. It has the same electron configuration as the noble gas argon.
Ionic Bond • Type of chemical bond • Electron transfers lead to forces holding atoms together • Binds opposite charged ions together • Formed by a METAL and a NONMETAL (or a group) • Examples: Na. Cl (Sodium Chloride), Na 2 CO 3 (Sodium Carbonate) **To determine is an element is a metal, nonmetal or metalloid, look at its placement on the periodic table.

Ionic Bonding • A bond forms when oppositely charged atoms are electrostatically attracted to one another as a result of the transfer of electrons

Classes of Ionic Bonds 1. Oxides • Compounds with ionic bonds between a metal and oxygen • Example: Mg. O (Magnesium Oxide) 2. Salts • Generic name for most ionic compounds • Examples: Na. Cl (Sodium Chloride), Zn. I 2 (Zinc Iodide)

Intro to Ionic Names & Formulas • Monatomic Ions: one-atom ions 1) Cation • Positive ion formed by the loss of valence electrons • Atom loses electrons to have an octet like the previous noble gases • Naming: The cation name stays the same as the atom name • Example: Li Lithium Li+ Lithium Ion

Monatomic Ions 2) Anion • A Negative ion formed by the gain of electrons • Atoms gain electrons to achieve an octet • Naming: For the anion, add the suffix –ide to the root of the atom name • Example: Br bromine, Br - Bromide Ion

Oxidation Number (Oxidation State) • The charge of a monatomic ion • The number of electrons gained or lost to make an ion • Monatomic Ion Trends: • Group 1: Plus 1 charge • Group 2: Plus 2 charge • Group 17: Negative 1 charge • Group 16: Negative 2 charge • Group 15: Negative 3 charge

Practice: Write the symbol, name of the ion, and determine if it’s a cation or anion. 1. An iodine atom gains one electron 2. A strontium atom loses two electrons 3. A sulfur atom gains two electrons 4. An aluminum atom loses three electrons

Practice: Atoms that tend to gain a noble gas configuration by LOSING valence electrons are A. Metals B. Nonmetals C. Noble Gases D. Representative Elements

Polyatomic Ions • Polyatomic Ions: • Ions made up of more than one atom • Charge applies to the whole group • Never change the subscripts of atoms within the ion • Example: CO 32 - (Carbonate) • Oxyanion: • Ion with a nonmetal and one or more oxygen atoms

Polyatomic Ion Trends Naming Element: S Anion: S 2 SO 52 SO 42 SO 32 SO 22 - Sulfur Sulfide Persulfate Sulfite Hyposulfite (+1 oxygen) (-2 oxygen)

Ionic Bonds • Ion Bond: Metal and Nonmetal Or Positive Ion and Negative Ion • When atoms transfer electrons, the atoms become bonded and form ionic compounds • Although they are composed of charged ions, ionic compounds are electrically neutral (positive charge = negative charge).

Ionic Nomenclature Writing Ionic Compound Formulas: 1. Write the cation and anion formulas with charges 2. Balance the charges with subscripts if necessary 3. Use parentheses around polyatomic ions that need a subscript added 4. Write the final neutral formula without charges

Practice: • Potassium Permanganate: K+ Mn. O 4 - KMn. O 4 • Aluminum Oxide: Al 3+ O 2 - add Al 3+ O 2 - Al 2 O 3 • Copper (II) Hydroxide: Cu 2+ OH- add OH- Cu(OH)2

More Practice: • Sodium Phosphate: Na 3 PO 4 • Iron (III) Sulfate: Fe 2(SO 4)3 • Calcium Manganate: Ca. Mn. O 4

Naming Ionic Compounds 1. Cation name written first 2. Anion name written second **Reminder: Use the roman numerals for most cations

Practice: • Li 3 PO 4 -- lithium phosphate • Fe(Cl. O 4)2 -- iron (II) perchlorate • Na 2 SO 4 – sodium sulfate

More Practice: • (NH 4)2 S Ammonium Sulfide • Ag. C 2 H 3 O 2 Silver Acetate • Cu. Cl Copper (I) Chloride • Li. HCO 3 Lithium Bicarbonate
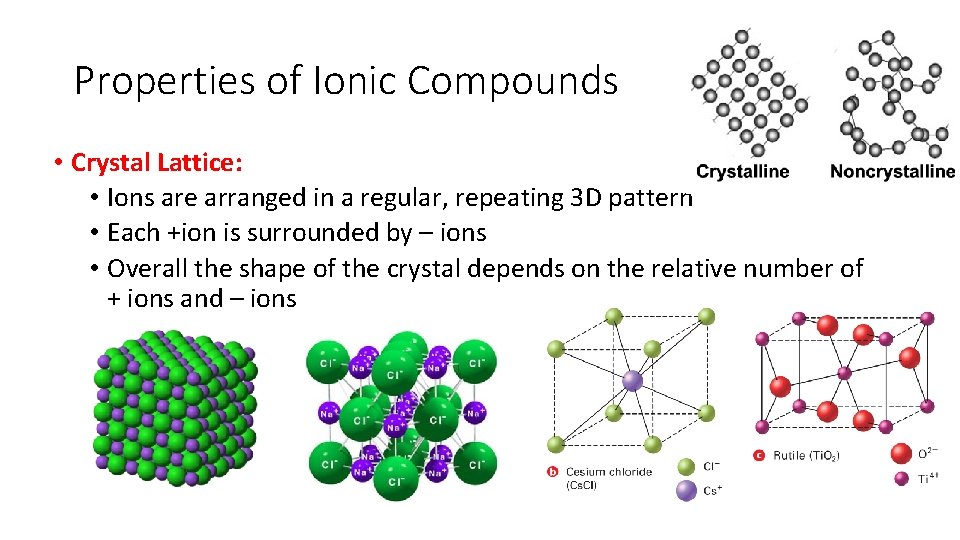
Properties of Ionic Compounds • Crystal Lattice: • Ions are arranged in a regular, repeating 3 D pattern • Each +ion is surrounded by – ions • Overall the shape of the crystal depends on the relative number of + ions and – ions

Crystal Structure
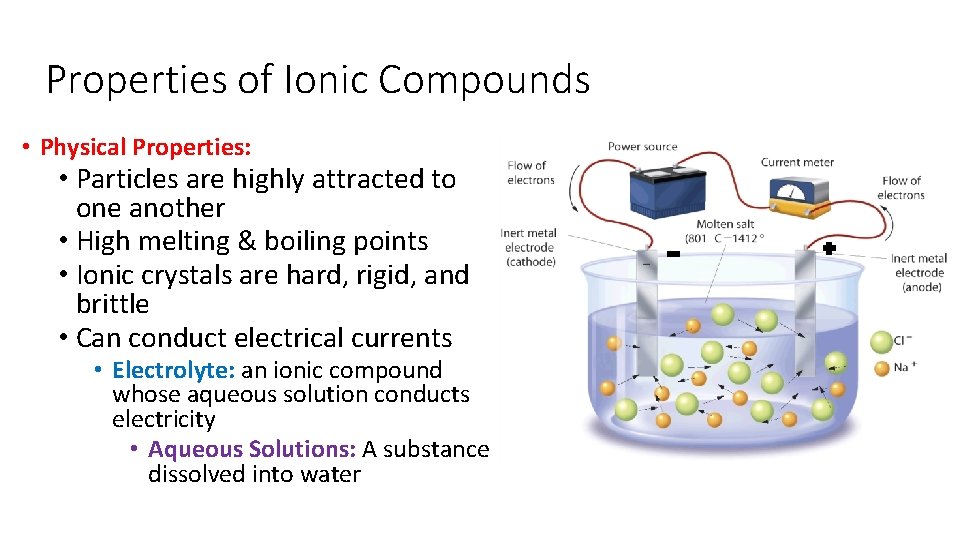
Properties of Ionic Compounds • Physical Properties: • Particles are highly attracted to one another • High melting & boiling points • Ionic crystals are hard, rigid, and brittle • Can conduct electrical currents • Electrolyte: an ionic compound whose aqueous solution conducts electricity • Aqueous Solutions: A substance dissolved into water
- Slides: 27