Ch 7 Chemical Formulas and Compounds 7 4





- Slides: 11

Ch. 7: Chemical Formulas and Compounds 7. 4 DETERMINING CHEMICAL FORMULAS

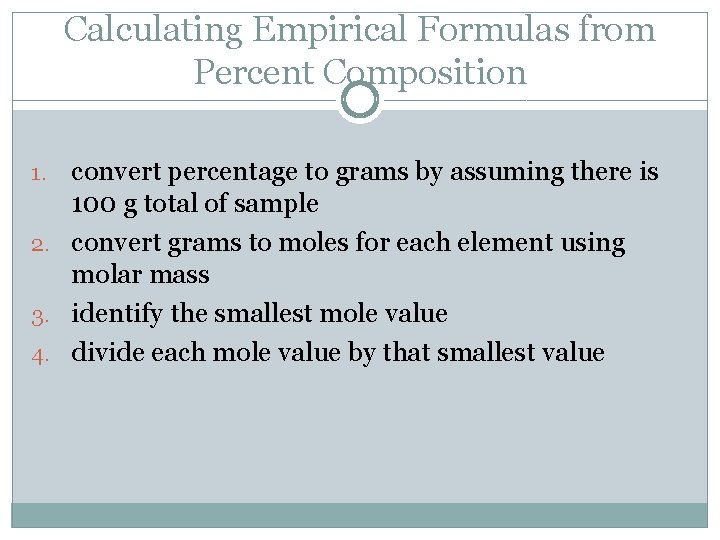
Empirical Formula �formula containing the simplest whole-number ratio of atoms �not necessarily the CORRECT molecular formula �Why? �Ex. BH 3 and B 2 H 6 have same empirical formula have different molecular formulas

Calculating Empirical Formulas from Percent Composition convert percentage to grams by assuming there is 100 g total of sample 2. convert grams to moles for each element using molar mass 3. identify the smallest mole value 4. divide each mole value by that smallest value 1.

Example 1 �Quantitative analysis shows that a compound contains 32. 38% Na, 22. 65% S, and 44. 99% O. Find the empirical formula. �Assuming you have 100 g of sample total 32. 38 g Na 22. 65 g S 44. 99 g O

Example 1 �Convert each of those to moles / 0. 7064 ≈ 2 / 0. 7064 ≈ 1 / 0. 7064 ≈ 4 �Divide by the smallest Na 2 SO 4 sodium sulfate

Calculating Empirical Formula from mass composition �don’t have to assume you have 100 g since you have an actual amount �follow all other steps the same way

Example 2 �Analysis of a 10. 150 g sample of a compound containing only P and O, is known to contain 4. 433 g of P. Find the empirical formula. �Find the mass of all components mtotal = m. P + m. O so m. O = mtotal – m. P mass of O : 10. 150 - 4. 433 = 5. 717 g

Example 2 �Convert all mass values to moles using molar mass / 0. 1431 ≈ 1 / 0. 1431 ≈ 2. 5 �Divide by the smallest mole value �To get rid of the decimal, multiply by integer P 2 O 5 diphosphorus pentoxide

Molecular Formulas �the molecular formula is the actual formula for a compound �could be same as the empirical formula but doesn’t have to be same �to find molecular formula: need empirical formula need actual molar mass (or formula mass) �compare the molar mass of empirical formula to actual molar mass

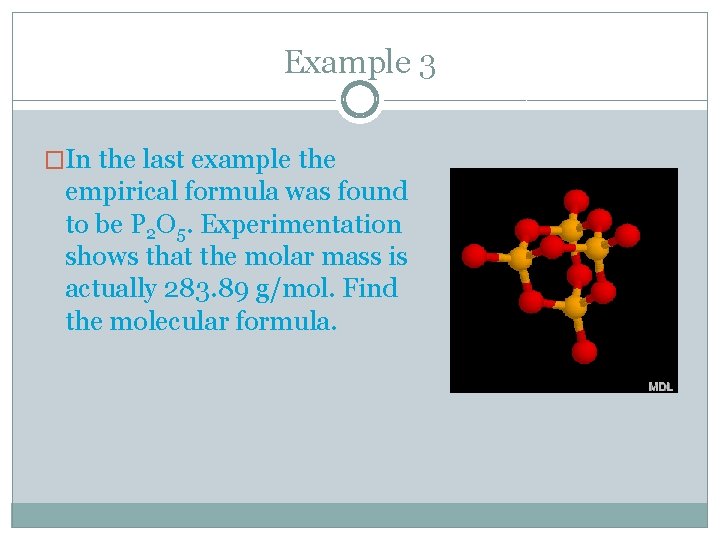
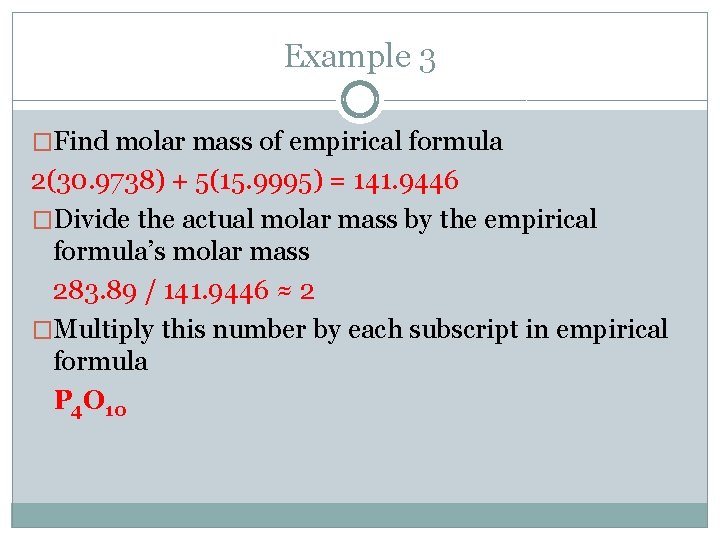
Example 3 �In the last example the empirical formula was found to be P 2 O 5. Experimentation shows that the molar mass is actually 283. 89 g/mol. Find the molecular formula.

Example 3 �Find molar mass of empirical formula 2(30. 9738) + 5(15. 9995) = 141. 9446 �Divide the actual molar mass by the empirical formula’s molar mass 283. 89 / 141. 9446 ≈ 2 �Multiply this number by each subscript in empirical formula P 4 O 10