Ch 7 3 Using Chemical Formulas The Mass







- Slides: 18

Ch 7. 3 Using Chemical Formulas

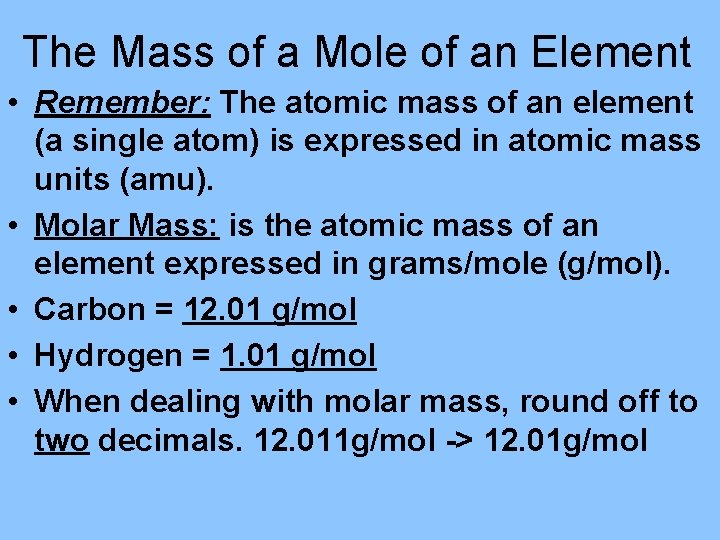
The Mass of a Mole of an Element • Remember: The atomic mass of an element (a single atom) is expressed in atomic mass units (amu). • Molar Mass: is the atomic mass of an element expressed in grams/mole (g/mol). • Carbon = 12. 01 g/mol • Hydrogen = 1. 01 g/mol • When dealing with molar mass, round off to two decimals. 12. 011 g/mol -> 12. 01 g/mol

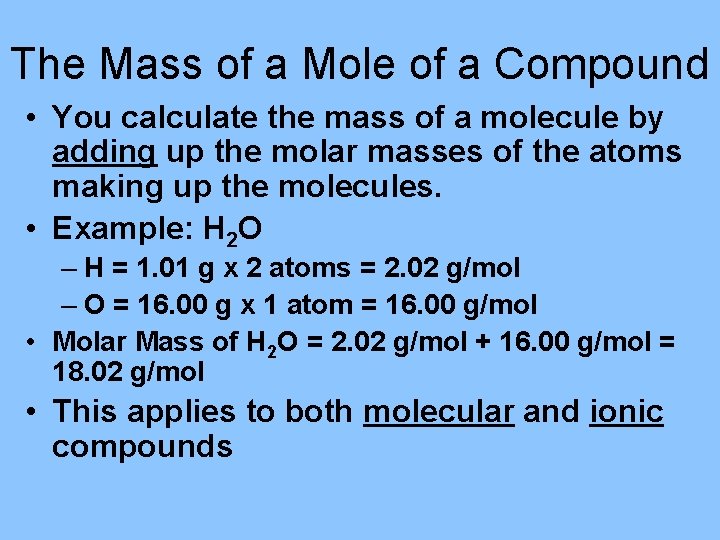
The Mass of a Mole of a Compound • You calculate the mass of a molecule by adding up the molar masses of the atoms making up the molecules. • Example: H 2 O – H = 1. 01 g x 2 atoms = 2. 02 g/mol – O = 16. 00 g x 1 atom = 16. 00 g/mol • Molar Mass of H 2 O = 2. 02 g/mol + 16. 00 g/mol = 18. 02 g/mol • This applies to both molecular and ionic compounds

• Find the molar mass of PCl 3 – P = 30. 97 g x 1 atom = 30. 97 g/mol – Cl = 35. 45 g x 3 atoms = 106. 35 g/mol – PCl 3 = 30. 97 g + 106. 35 g = 137. 32 g/mol • What is the molar mass of Sodium Hydrogen Carbonate (Na. HCO 3) ? – Na = 22. 99 g x 1 atom = 22. 99 g/mol – H = 1. 01 g x 1 atom = 1. 01 g/mol – C = 12. 01 g x 1 atom = 12. 01 g/mol – O = 16. 00 g x 3 atoms = 48. 00 g/mol – Na. HCO 3 = 22. 99 + 1. 01 + 12. 01 + 48. 00 = 84. 01 g/mol

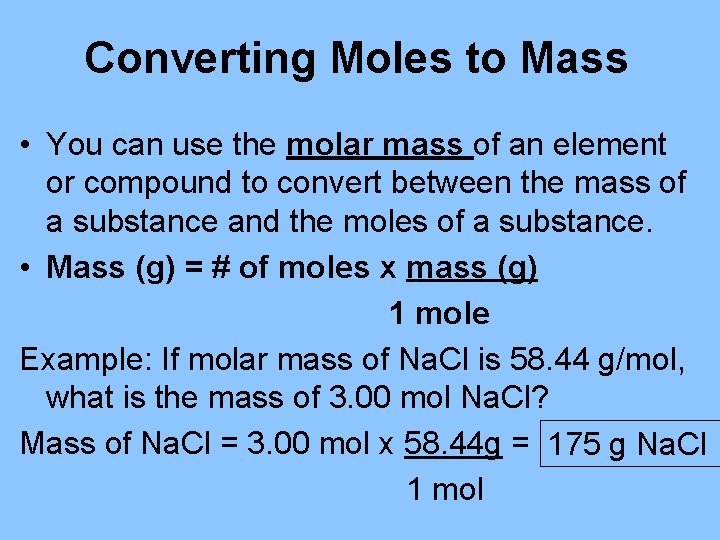
Converting Moles to Mass • You can use the molar mass of an element or compound to convert between the mass of a substance and the moles of a substance. • Mass (g) = # of moles x mass (g) 1 mole Example: If molar mass of Na. Cl is 58. 44 g/mol, what is the mass of 3. 00 mol Na. Cl? Mass of Na. Cl = 3. 00 mol x 58. 44 g = 175 g Na. Cl 1 mol

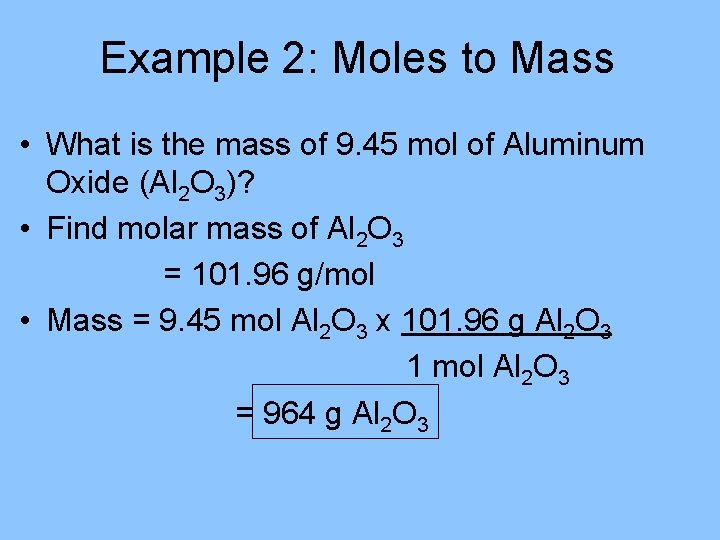
Example 2: Moles to Mass • What is the mass of 9. 45 mol of Aluminum Oxide (Al 2 O 3)? • Find molar mass of Al 2 O 3 = 101. 96 g/mol • Mass = 9. 45 mol Al 2 O 3 x 101. 96 g Al 2 O 3 1 mol Al 2 O 3 = 964 g Al 2 O 3

Converting Mass to Moles • You can invert the conversion factor to find moles when given the mass. • Moles = mass (g) x 1 mole mass (g) Example: If molar mass of Na 2 SO 4 142. 05 g/mol, how many moles is 10. 0 g of Na 2 SO 4? Moles of Na 2 SO 4 = 10. 0 g x 1 mol = 142. 05 g = 0. 0704 mol Na 2 SO 4

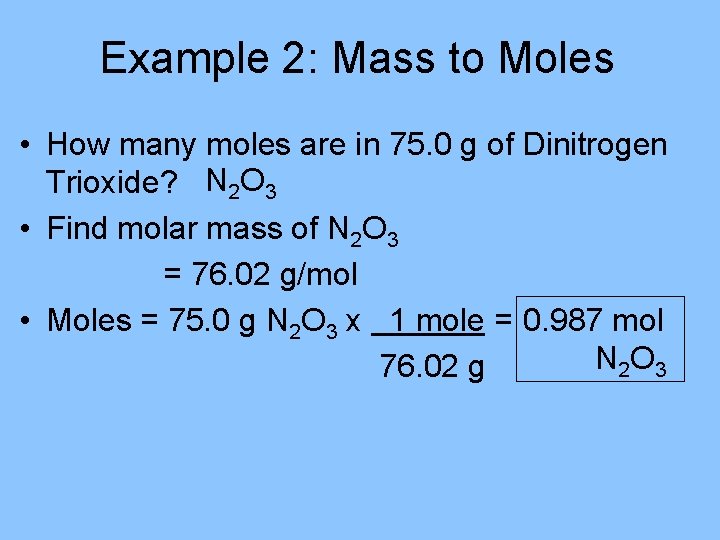
Example 2: Mass to Moles • How many moles are in 75. 0 g of Dinitrogen Trioxide? N 2 O 3 • Find molar mass of N 2 O 3 = 76. 02 g/mol • Moles = 75. 0 g N 2 O 3 x 1 mole = 0. 987 mol N 2 O 3 76. 02 g

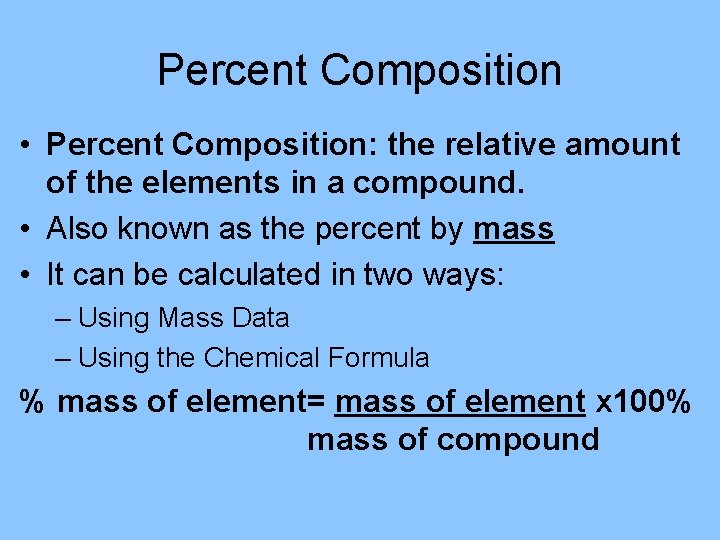
Percent Composition • Percent Composition: the relative amount of the elements in a compound. • Also known as the percent by mass • It can be calculated in two ways: – Using Mass Data – Using the Chemical Formula % mass of element= mass of element x 100% mass of compound

Example • When a 13. 60 g sample of a compound containing Mg and O is decomposed, 5. 40 g O is obtained. What is the % composition of this compound? Mass of compound: 13. 60 g Mass of oxygen: 5. 40 g O Mass of magnesium: 13. 60 g - 5. 40 g = 8. 20 g Mg % Mg = 8. 20 g Mg x 100% = 60. 3% 13. 60 g % O = 5. 40 g O x 100% = 39. 7% 13. 60 g

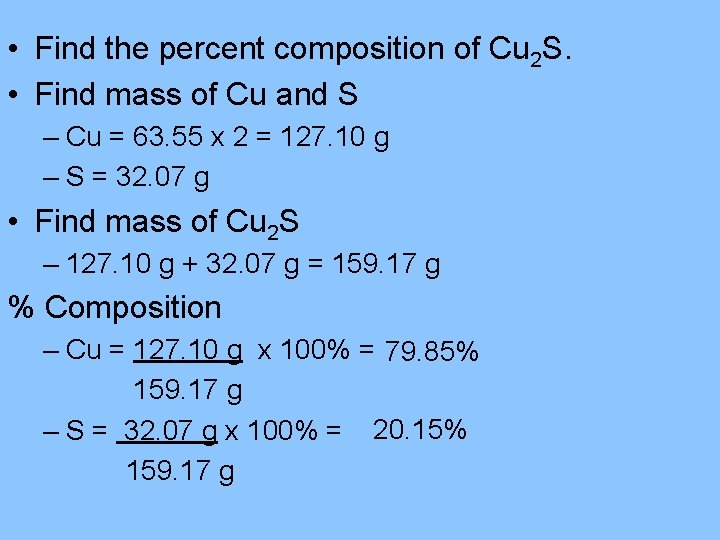
• Find the percent composition of Cu 2 S. • Find mass of Cu and S – Cu = 63. 55 x 2 = 127. 10 g – S = 32. 07 g • Find mass of Cu 2 S – 127. 10 g + 32. 07 g = 159. 17 g % Composition – Cu = 127. 10 g x 100% = 79. 85% 159. 17 g – S = 32. 07 g x 100% = 20. 15% 159. 17 g

Homework • 7. 3 pg 253 #30 -33

Ch 7. 4 Determining Chemical Formulas

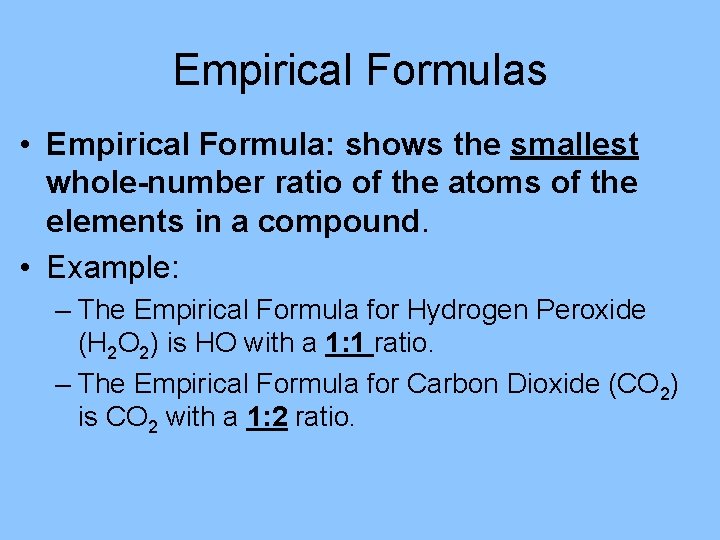
Empirical Formulas • Empirical Formula: shows the smallest whole-number ratio of the atoms of the elements in a compound. • Example: – The Empirical Formula for Hydrogen Peroxide (H 2 O 2) is HO with a 1: 1 ratio. – The Empirical Formula for Carbon Dioxide (CO 2) is CO 2 with a 1: 2 ratio.

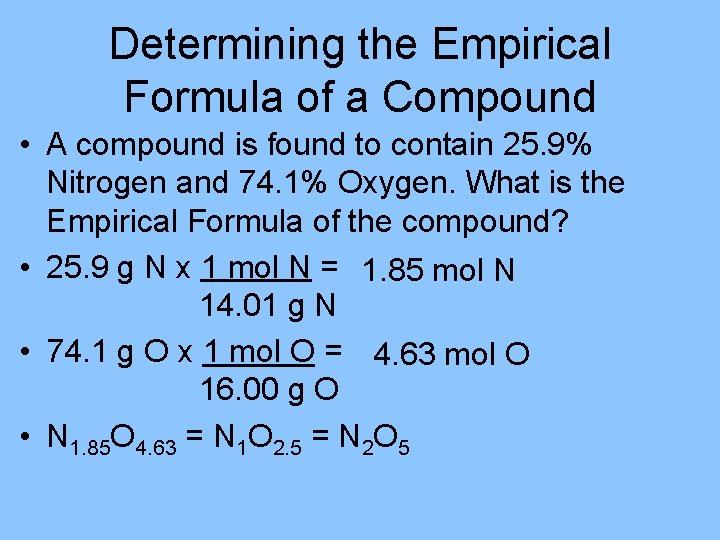
Determining the Empirical Formula of a Compound • A compound is found to contain 25. 9% Nitrogen and 74. 1% Oxygen. What is the Empirical Formula of the compound? • 25. 9 g N x 1 mol N = 1. 85 mol N 14. 01 g N • 74. 1 g O x 1 mol O = 4. 63 mol O 16. 00 g O • N 1. 85 O 4. 63 = N 1 O 2. 5 = N 2 O 5

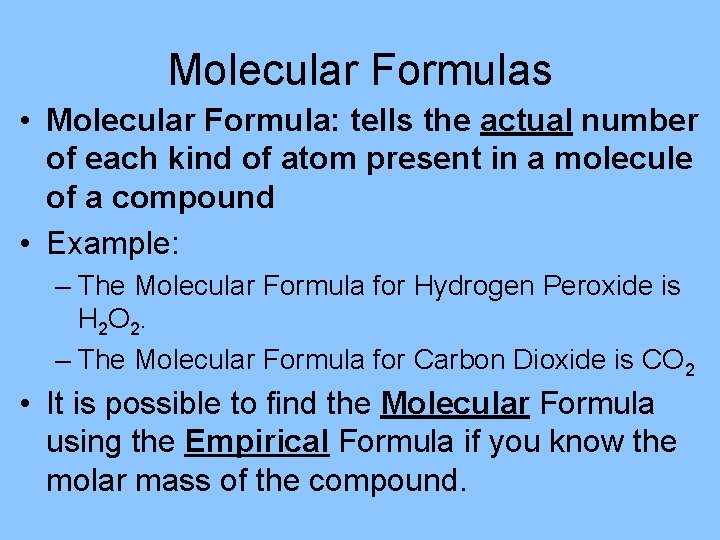
Molecular Formulas • Molecular Formula: tells the actual number of each kind of atom present in a molecule of a compound • Example: – The Molecular Formula for Hydrogen Peroxide is H 2 O 2. – The Molecular Formula for Carbon Dioxide is CO 2 • It is possible to find the Molecular Formula using the Empirical Formula if you know the molar mass of the compound.

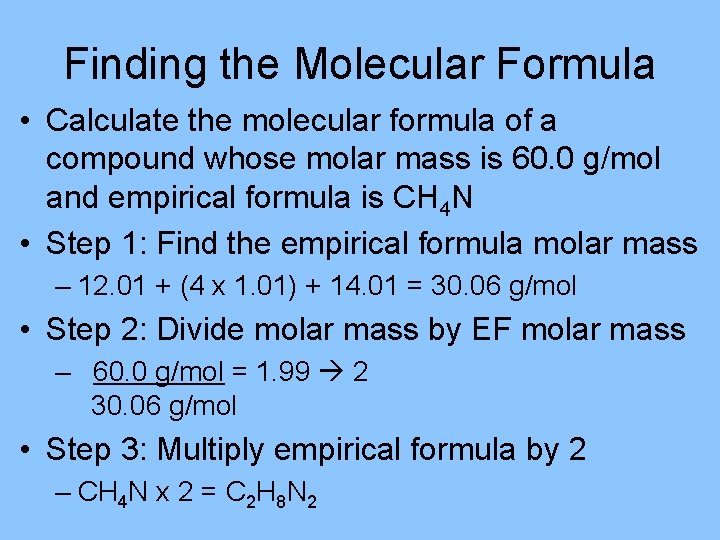
Finding the Molecular Formula • Calculate the molecular formula of a compound whose molar mass is 60. 0 g/mol and empirical formula is CH 4 N • Step 1: Find the empirical formula molar mass – 12. 01 + (4 x 1. 01) + 14. 01 = 30. 06 g/mol • Step 2: Divide molar mass by EF molar mass – 60. 0 g/mol = 1. 99 2 30. 06 g/mol • Step 3: Multiply empirical formula by 2 – CH 4 N x 2 = C 2 H 8 N 2

Homework • 7. 4 pg 253 #36 -38