Ch 6 Molecular Structure I Lewis Diagrams p


- Slides: 12

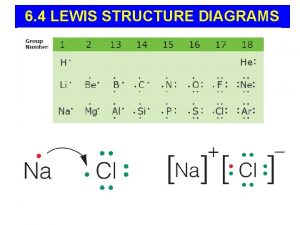
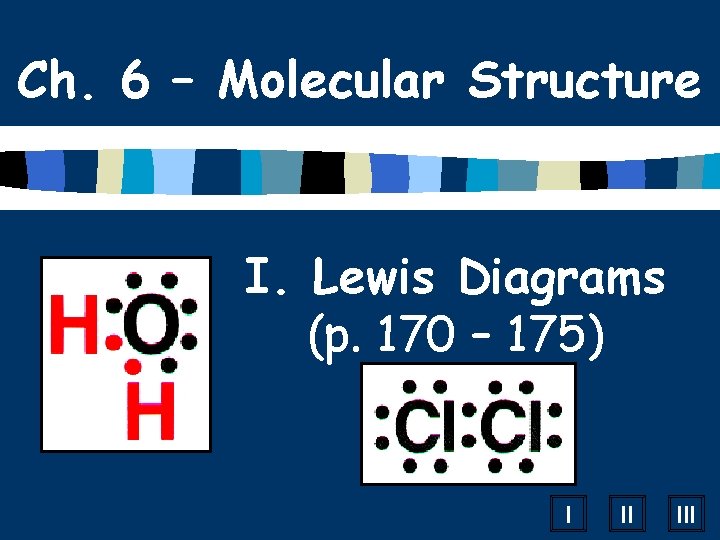
Ch. 6 – Molecular Structure I. Lewis Diagrams (p. 170 – 175) I II III

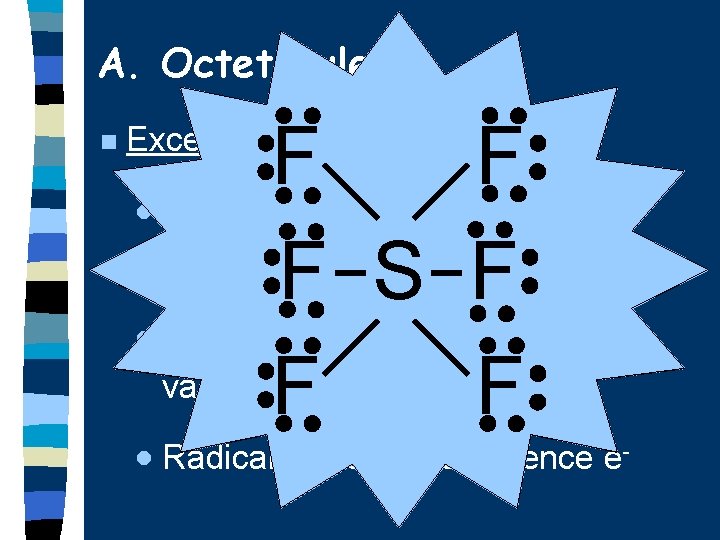
A. Octet Rule n Remember… · Most atoms form bonds in order to have 8 valence electrons.

A. Octet Rule n F F · Hydrogen 2 valence e F B F · Groups F 1, 2, 3 get 2, 4, 6 valence e S F H N O O H · Expanded octet more than 8 F Very unstable!! valence e (e. g. S, P, Xe) F F Exceptions: - - · Radicals odd # of valence e- -

B. Drawing Lewis Diagrams n Find total # of valence e-. n Arrange atoms - singular atom is usually in the middle. n Form bonds between atoms (2 e-). n Distribute remaining e- to give each atom an octet (recall exceptions). n If there aren’t enough e- to go around, form double or triple bonds.

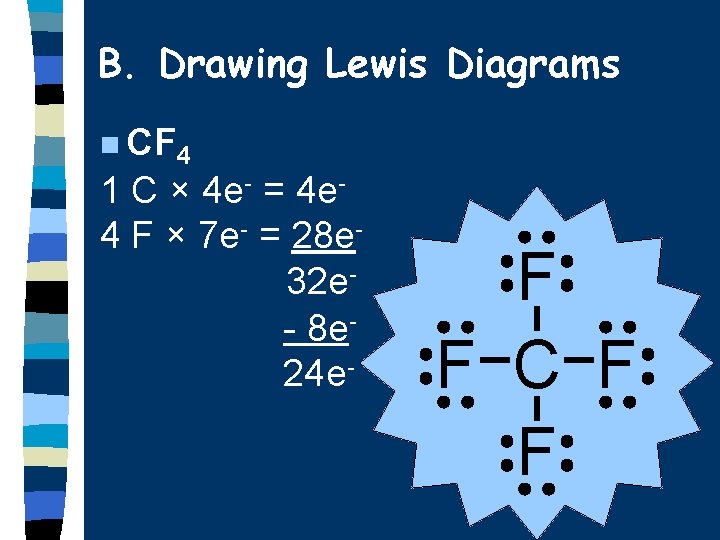
B. Drawing Lewis Diagrams n CF 4 1 C × 4 e- = 4 e 4 F × 7 e- = 28 e 32 e- 8 e 24 e- F F C F F

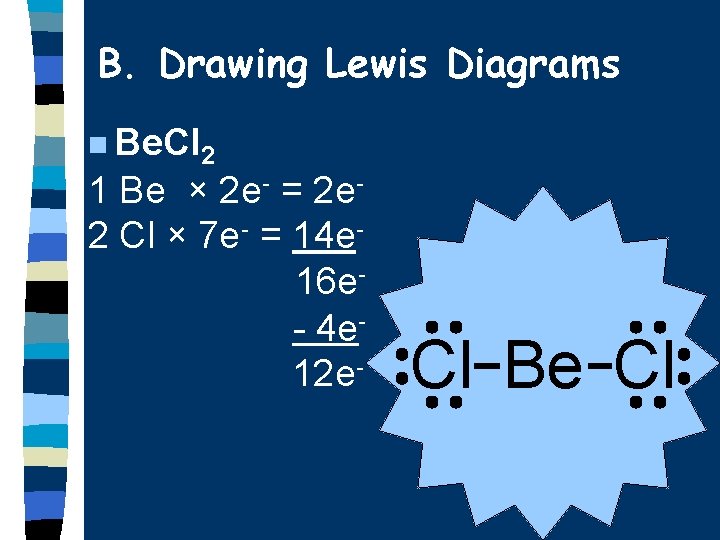
B. Drawing Lewis Diagrams n Be. Cl 2 1 Be × 2 e- = 2 e 2 Cl × 7 e- = 14 e 16 e- 4 e 12 e- Cl Be Cl

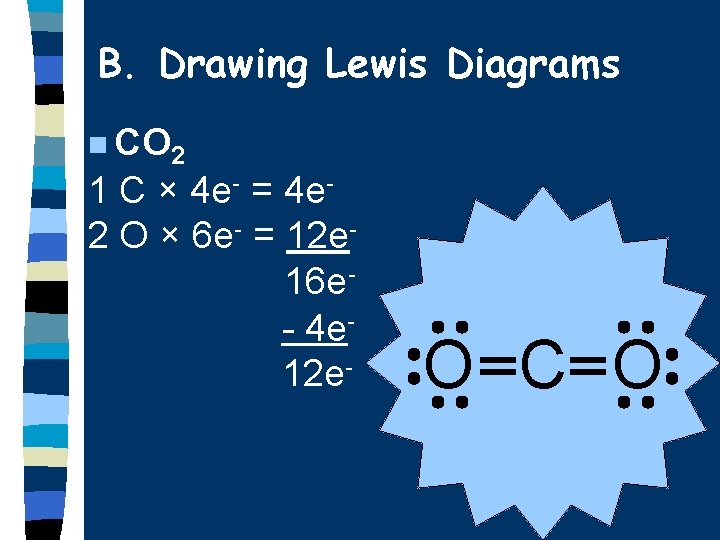
B. Drawing Lewis Diagrams n CO 2 1 C × 4 e- = 4 e 2 O × 6 e- = 12 e 16 e- 4 e 12 e- O C O

C. Polyatomic Ions n To find total # of valence e-: · Add 1 e- for each negative charge. · Subtract 1 e- for each positive charge. n Place brackets around the ion and label the charge.

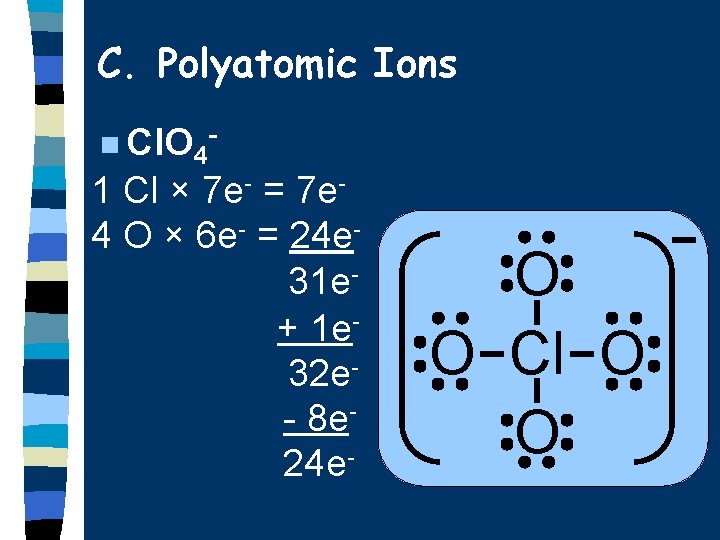
C. Polyatomic Ions n Cl. O 4 - 1 Cl × 7 e- = 7 e 4 O × 6 e- = 24 e 31 e+ 1 e 32 e- 8 e 24 e- O O Cl O O

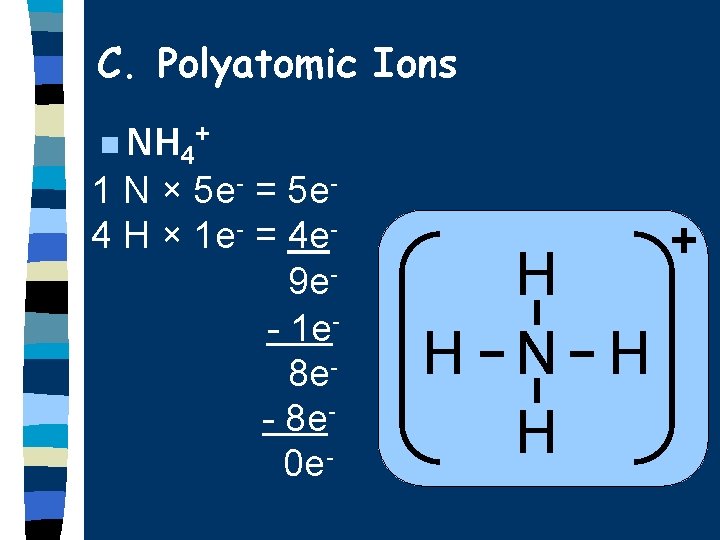
C. Polyatomic Ions n NH 4+ 1 N × 5 e- = 5 e 4 H × 1 e- = 4 e 9 e- 1 e 8 e- 8 e 0 e- H H N H H

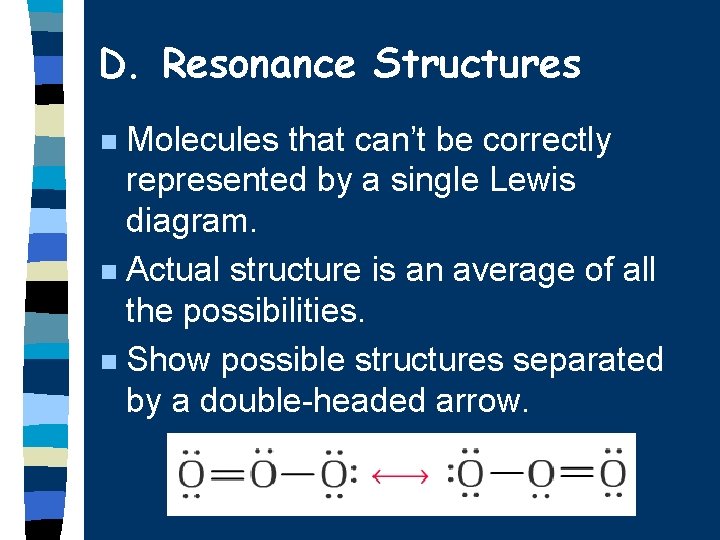
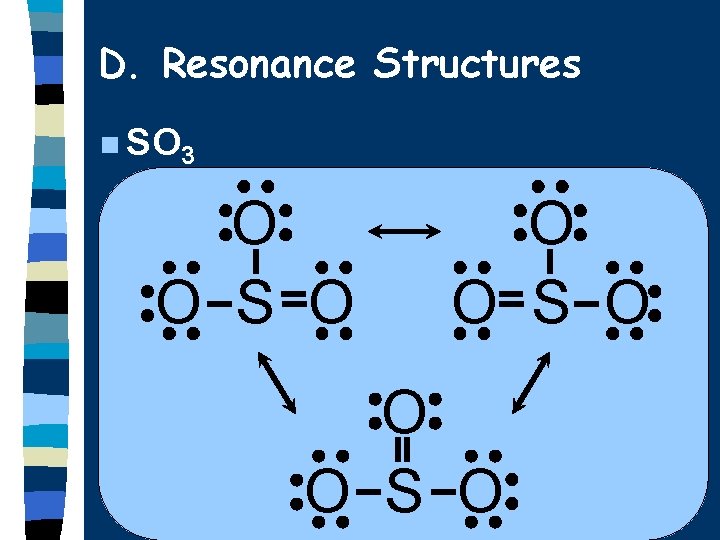
D. Resonance Structures Molecules that can’t be correctly represented by a single Lewis diagram. n Actual structure is an average of all the possibilities. n Show possible structures separated by a double-headed arrow. n

D. Resonance Structures n SO 3 O O S O
 Melting and boiling point of oxygen
Melting and boiling point of oxygen Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Molecular shape for ch2o
Molecular shape for ch2o Theory of structures
Theory of structures Pcl4br lewis structure
Pcl4br lewis structure Electron dot structure of ethene
Electron dot structure of ethene O3 molecular geometry
O3 molecular geometry Nocl molecular shape
Nocl molecular shape The interaction diagrams, use case diagrams are called as
The interaction diagrams, use case diagrams are called as Activity diagram if
Activity diagram if Draw a bohr model of carbon
Draw a bohr model of carbon Review bohr and lewis dot diagrams
Review bohr and lewis dot diagrams