Ch 6 Covalent Compounds What determines whether two







- Slides: 28

Ch 6 Covalent Compounds What determines whether two atoms will form a bond? n How can a hydrogen atom, which has one valence electron, bond with chlorine, which has seven valence electrons? n What happens in terms of energy after a hydrogen atom bonds with a chlorine atom? n

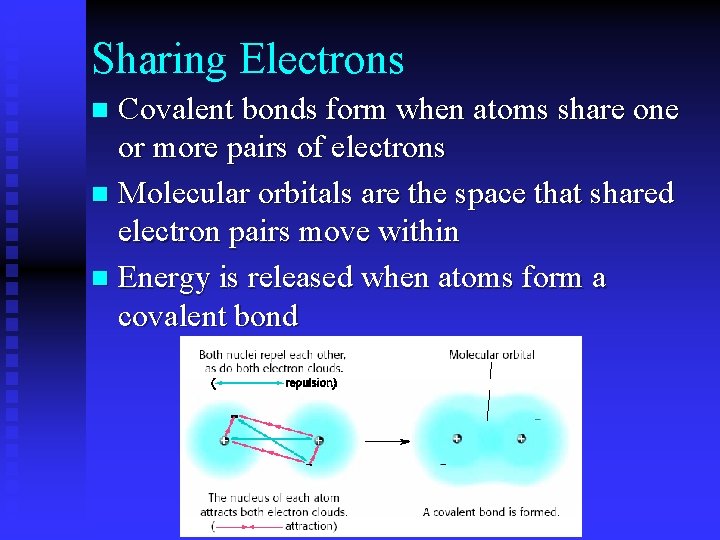
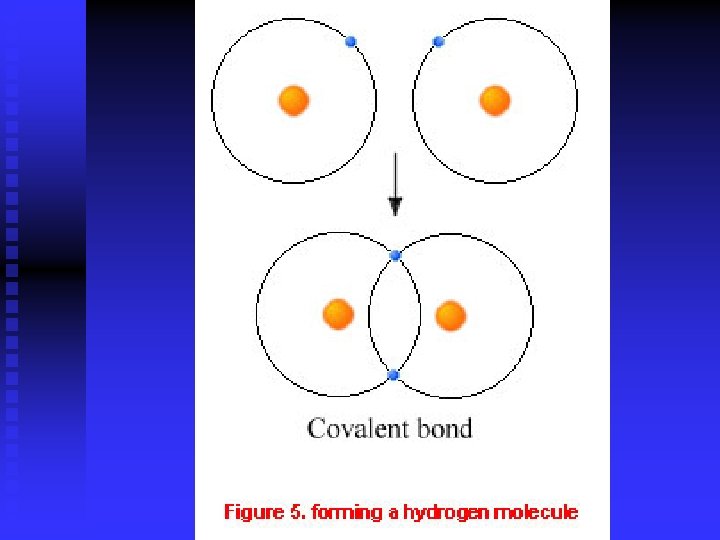
Sharing Electrons Covalent bonds form when atoms share one or more pairs of electrons n Molecular orbitals are the space that shared electron pairs move within n Energy is released when atoms form a covalent bond n

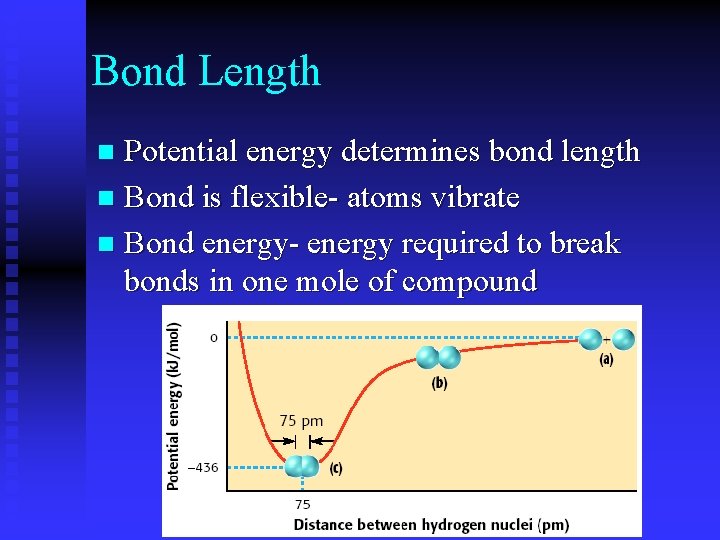
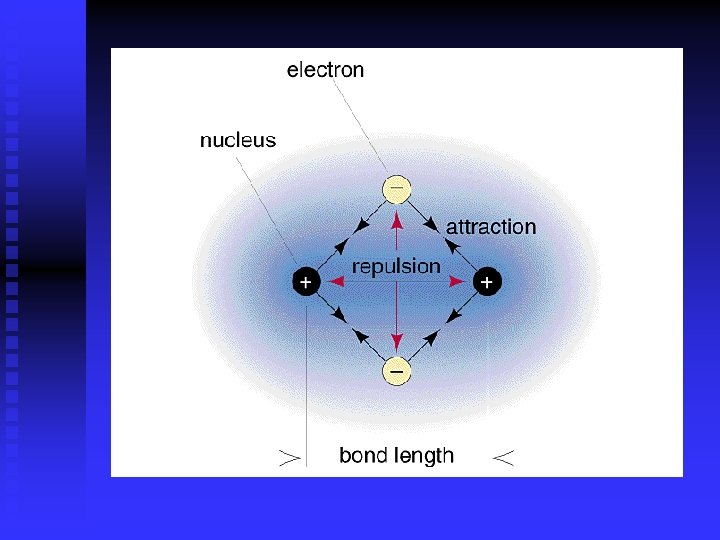
Bond Length Potential energy determines bond length n Bond is flexible- atoms vibrate n Bond energy- energy required to break bonds in one mole of compound n


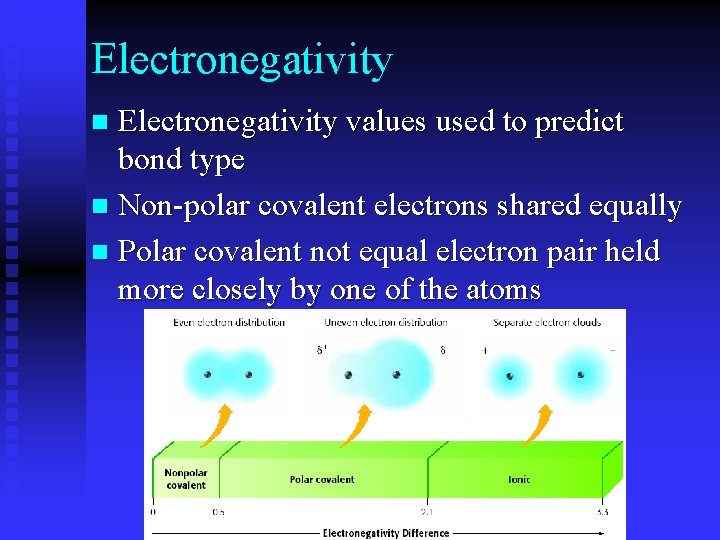
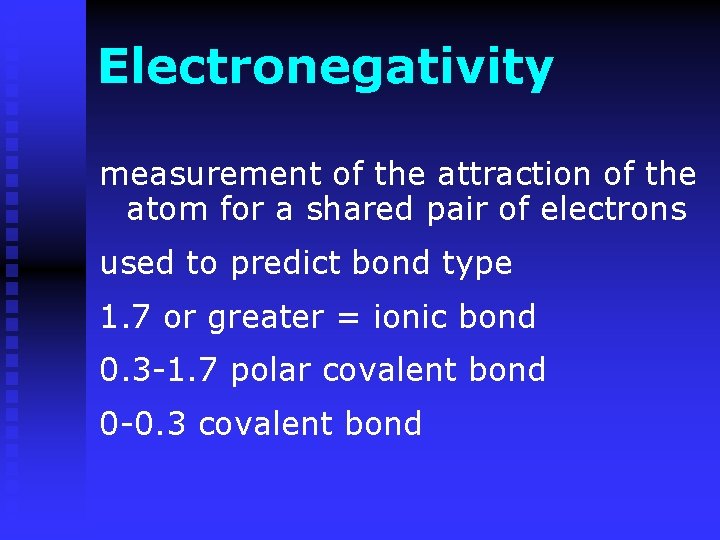
Electronegativity values used to predict bond type n Non-polar covalent electrons shared equally n Polar covalent not equal electron pair held more closely by one of the atoms n

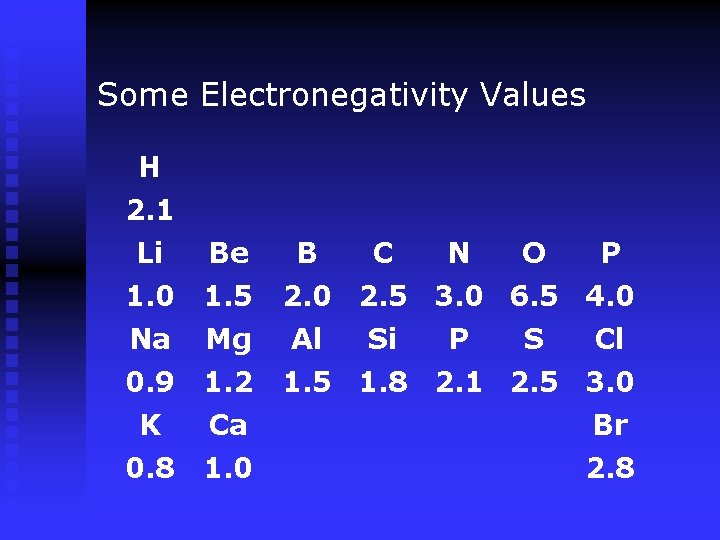
Some Electronegativity Values H 2. 1 Li Be B C N O P 1. 0 1. 5 Na Mg 0. 9 1. 2 1. 5 1. 8 2. 1 2. 5 3. 0 K Ca Br 0. 8 1. 0 2. 8 2. 0 2. 5 3. 0 6. 5 4. 0 Al Si P S Cl

Electronegativity measurement of the attraction of the atom for a shared pair of electrons used to predict bond type 1. 7 or greater = ionic bond 0. 3 -1. 7 polar covalent bond 0 -0. 3 covalent bond

Bonding between non-metals and non-metals. Therefore all atoms included have fairly high electronegativity and few vacancies in valence energy levels. When they bond, they gain electrons to achieve stable configuration. Hence, electrons are shared. Sharing produces low energy (stable) electron arrangements

Properties 1. Gases, liquids, or solids (made of molecules) 2. Low melting and boiling points 3. Poor electrical conductors in all phases 4. Many soluble in nonpolar liquids but not in water

Polar molecule Dipole – partial charges on ends n Greater electronegativity difference produces dipoles n Bond types classified by bond character n


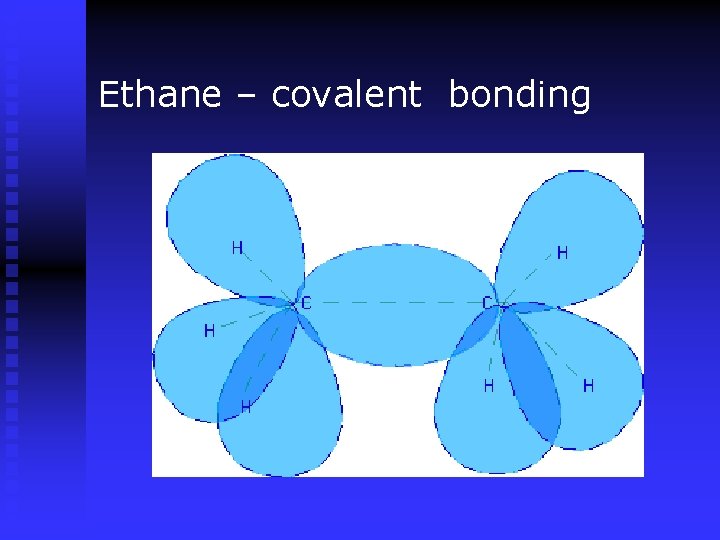
Ethane – covalent bonding

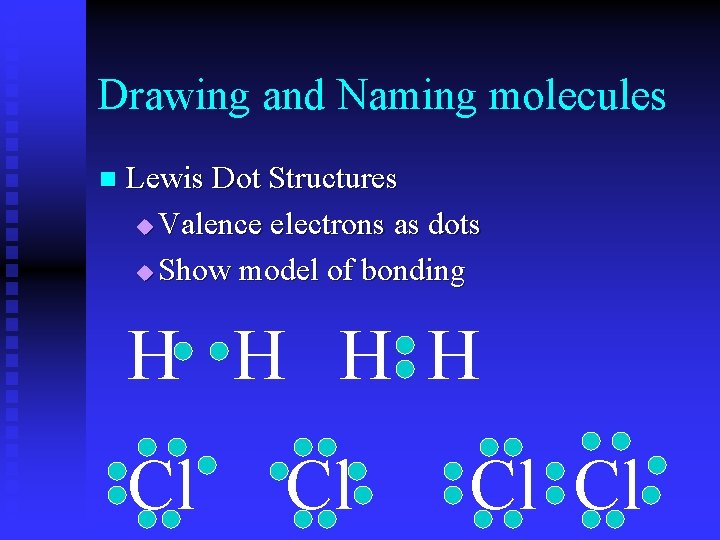
Drawing and Naming molecules n Lewis Dot Structures u Valence electrons as dots u Show model of bonding H H Cl Cl

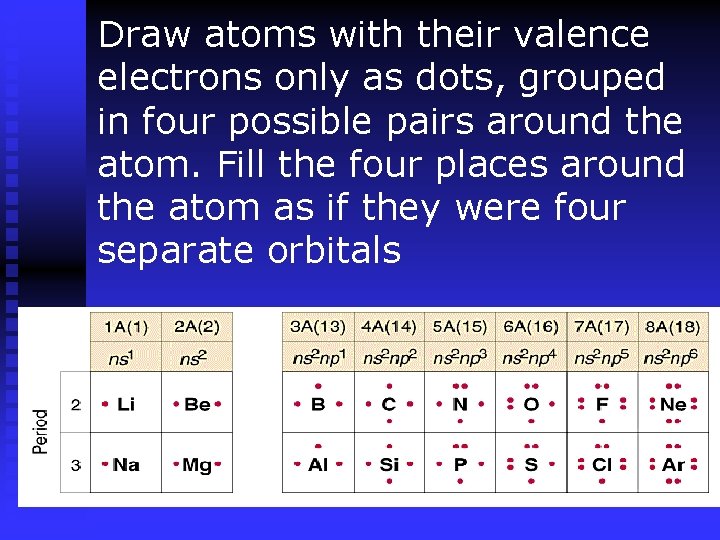
Draw atoms with their valence electrons only as dots, grouped in four possible pairs around the atom. Fill the four places around the atom as if they were four separate orbitals

Now combine atoms together to form molecules by pairing electrons without changing the total number of electrons. Make an 'octet' around each atom in this way (except Hydrogen which can only support 2 valence electrons

Octet ruleatoms form bonds to achieve a noble gas electron configuration. Each atom wants 8 electrons in its valence orbital 1. 2. 3. 4. How many atoms ? How many valence electrons ? Skeletal structure Where do dots go?

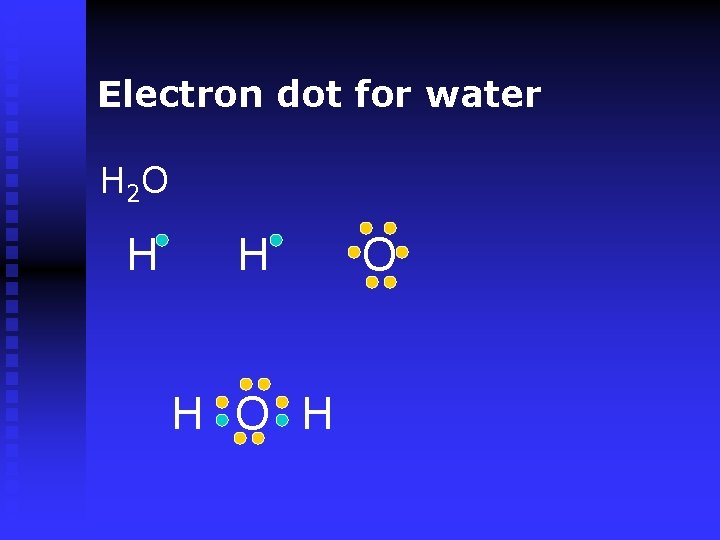
Electron dot for water H 2 O H H H O

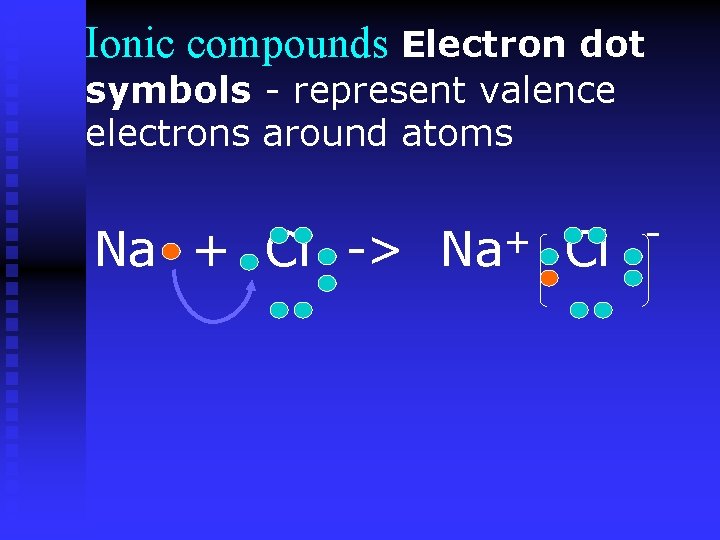
Ionic compounds Electron dot symbols - represent valence electrons around atoms Na + Cl -> + Na Cl -

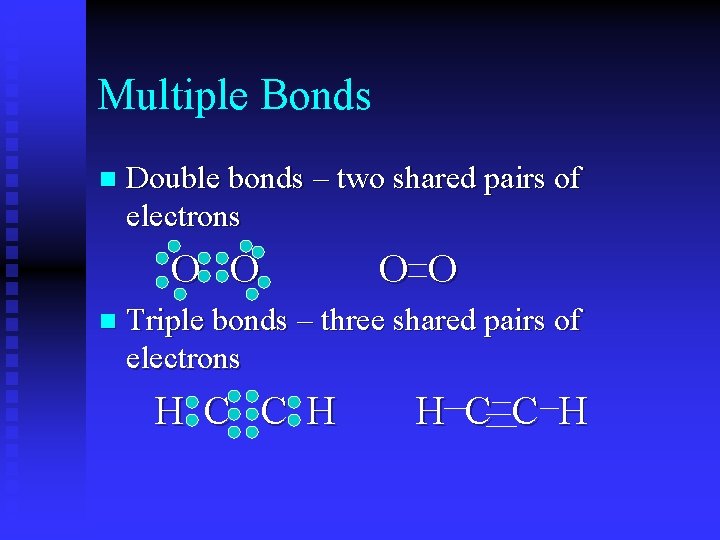
Multiple Bonds n Double bonds – two shared pairs of electrons O O n O O Triple bonds – three shared pairs of electrons H C C H

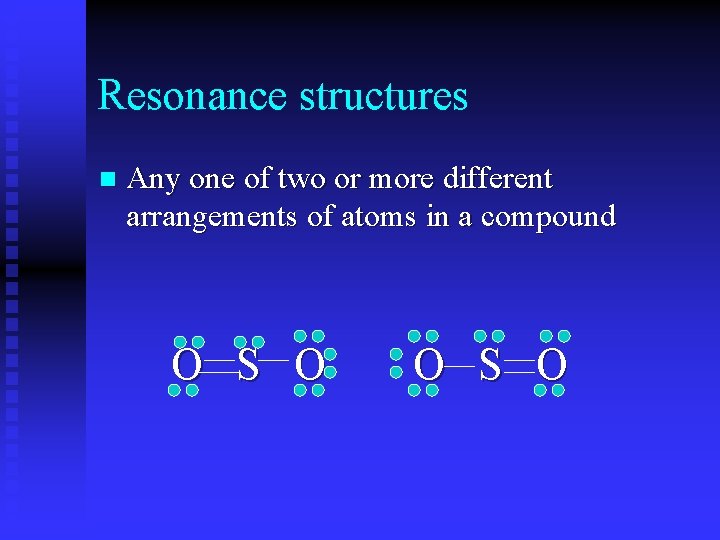
Resonance structures n Any one of two or more different arrangements of atoms in a compound O S O

Naming Covalent Compounds Prefixes indicate how many atoms are present n Second element ends in - ide n P 2 S 5 Diphosphorus pentasulfide

Prefixes Mono - 1 n Di - 2 n Tri - 3 n Tetra - 4 n Penta - 5 n

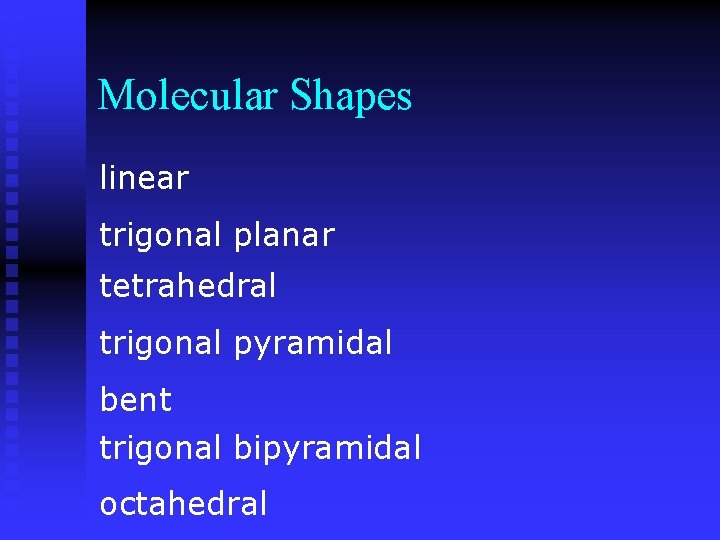
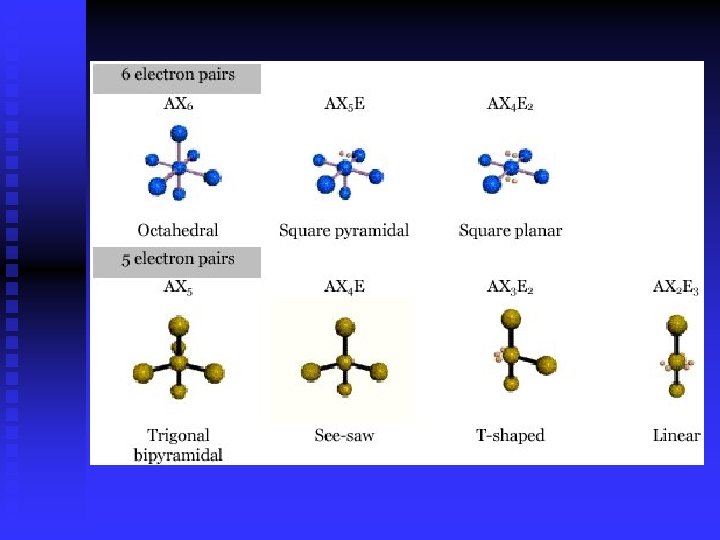
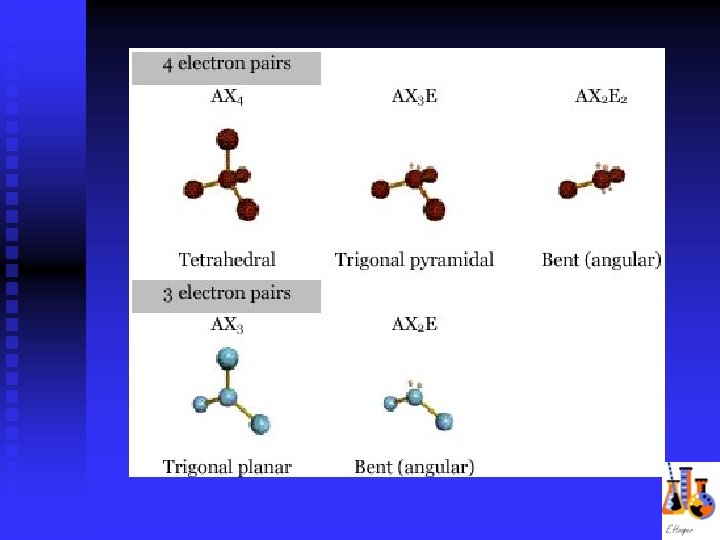
Molecular Shapes linear trigonal planar tetrahedral trigonal pyramidal bent trigonal bipyramidal octahedral

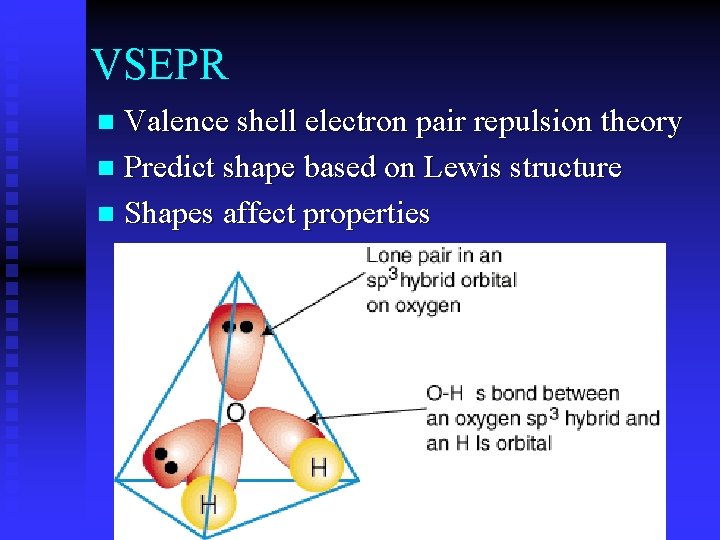
VSEPR Valence shell electron pair repulsion theory n Predict shape based on Lewis structure n Shapes affect properties n




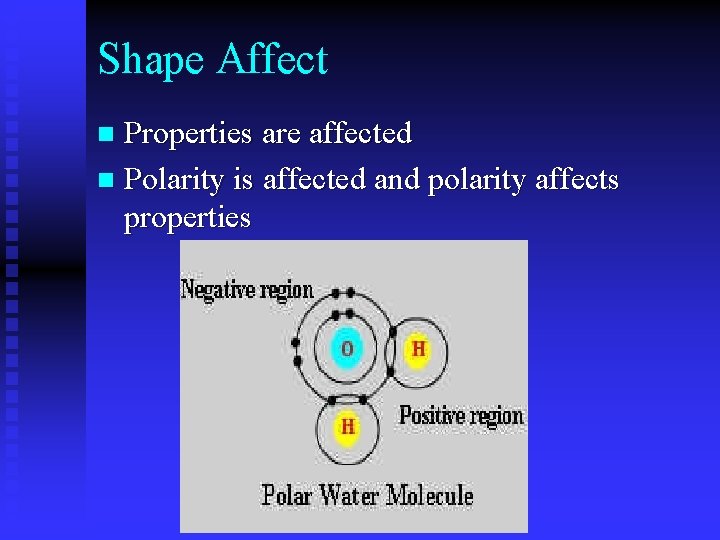
Shape Affect Properties are affected n Polarity is affected and polarity affects properties n