Ch 5 The Structure and Function of Macromolecules










- Slides: 46

Ch. 5 The Structure and Function of Macromolecules

The 4 Macromolecules o o o Carboyhydrates Lipids Proteins Nucleic acids may consist of thousands of covalently bonded atoms

Similarities: o chainlike molecules (polymers) n o o polymer -a long molecule with similar or identical building blocks linked by covalent bonds. small units – monomers All contain C, H, O

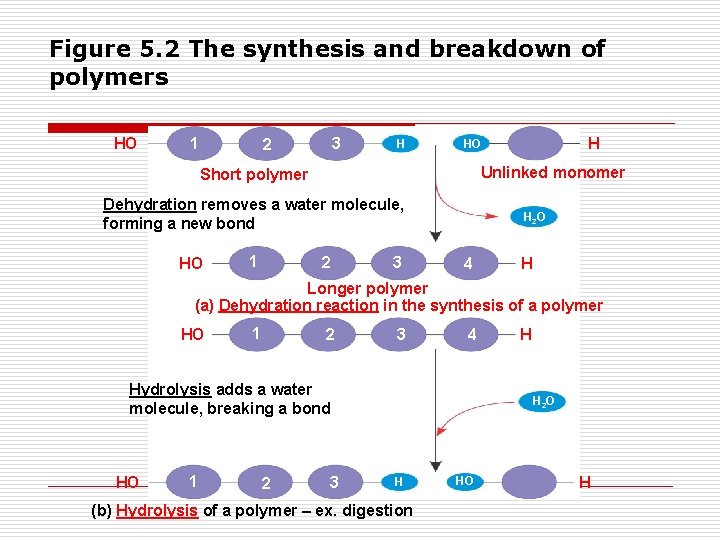
How are polymers made and broken down? o hydrolysis o dehydration synthesis reaction o Both involve water

Figure 5. 2 The synthesis and breakdown of polymers HO 1 3 2 H Unlinked monomer Short polymer Dehydration removes a water molecule, forming a new bond HO 1 H HO 2 3 H 2 O 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3 4 Hydrolysis adds a water molecule, breaking a bond HO 1 2 3 H H 2 O H (b) Hydrolysis of a polymer – ex. digestion HO H

Carbohydrates Sugars Cellulose Chitin

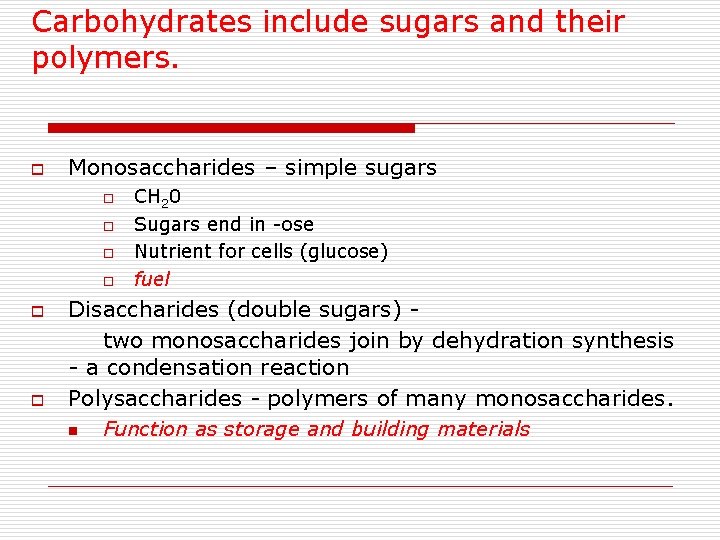
Carbohydrates include sugars and their polymers. o Monosaccharides – simple sugars o o o CH 20 Sugars end in -ose Nutrient for cells (glucose) fuel Disaccharides (double sugars) two monosaccharides join by dehydration synthesis - a condensation reaction Polysaccharides - polymers of many monosaccharides. n Function as storage and building materials

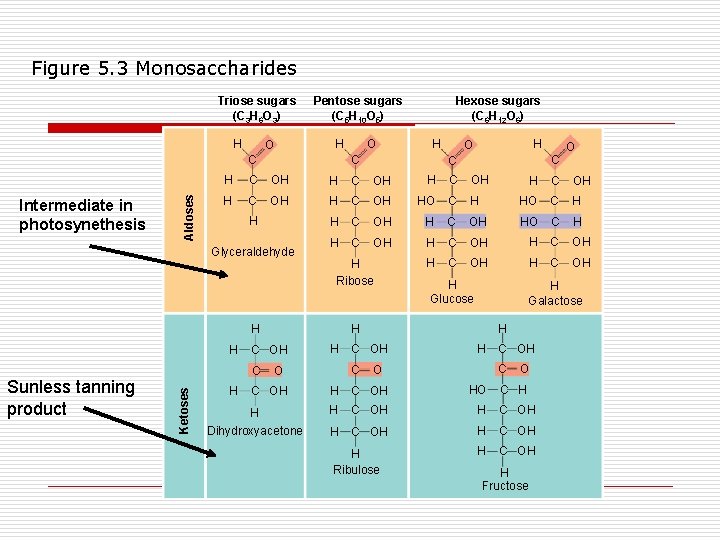
Figure 5. 3 Monosaccharides Triose sugars (C 3 H 6 O 3) H O Pentose sugars (C 5 H 10 O 5) H Intermediate in photosynethesis Aldoses C H O C C H C OH H C OH HO C H C OH H H Ribose H H H C OH H HO C H C OH HO C H H C OH H Glucose H Galactose C O H C OH C O O C OH HO H H C OH Dihydroxyacetone H C OH H H Ribulose O H H C OH C Ketoses H C Glyceraldehyde Sunless tanning product O Hexose sugars (C 6 H 12 O 6) C H H Fructose

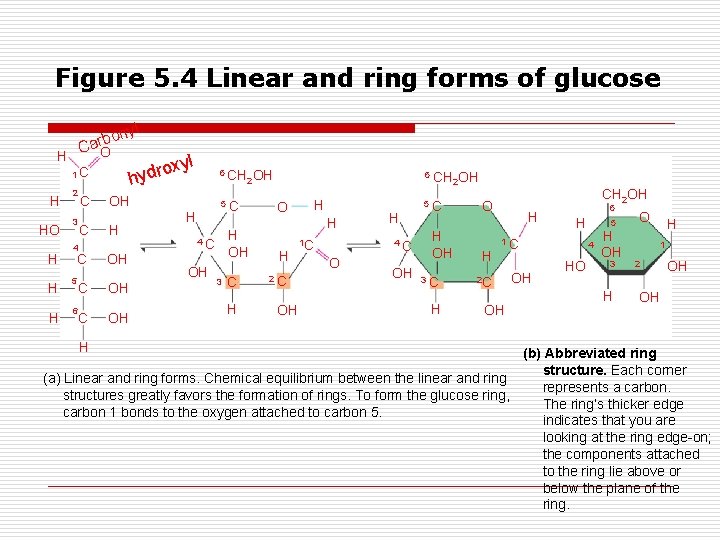
Figure 5. 4 Linear and ring forms of glucose l y bon r a C O H 1 C H HO H 2 3 C C 4 C H 5 H 6 C C H yl ox hydr OH H OH OH OH H 6 CH 2 OH 5 C H 4 C OH OH 3 C H 6 CH O H H H 2 C OH 1 C H 4 C O OH 2 OH 5 C H OH 3 C H CH 2 OH O H H 6 H 1 C 2 C OH OH 5 4 HO H OH 3 H O H 1 2 OH OH (b) Abbreviated ring structure. Each corner (a) Linear and ring forms. Chemical equilibrium between the linear and ring represents a carbon. structures greatly favors the formation of rings. To form the glucose ring, The ring’s thicker edge carbon 1 bonds to the oxygen attached to carbon 5. indicates that you are looking at the ring edge-on; the components attached to the ring lie above or below the plane of the ring.

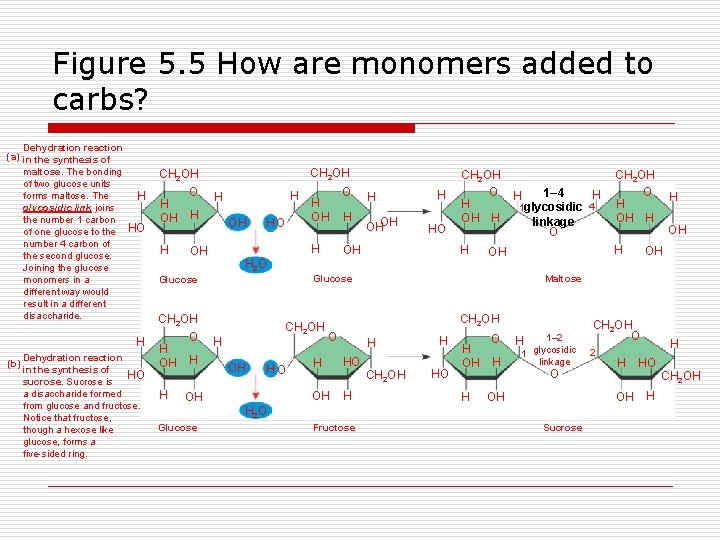
Figure 5. 5 How are monomers added to carbs? Dehydration reaction (a) in the synthesis of maltose. The bonding of two glucose units forms maltose. The glycosidic link joins the number 1 carbon of one glucose to the number 4 carbon of the second glucose. Joining the glucose monomers in a different way would result in a different disaccharide. H HO H CH 2 OH H H OH HO H 2 O CH 2 OH Dehydration reaction in the synthesis of HO sucrose. Sucrose is a disaccharide formed from glucose and fructose. Notice that fructose, though a hexose like glucose, forms a five-sided ring. H OH H OH CH 2 OH HO OH O H H OHOH H HO CH 2 OH O H OH H 1– 4 linkage O HO OH H CH 2 OH H HO H OH H O H 1– 2 H glycosidic 1 linkage O OH Fructose OH OH CH 2 OH O 2 H OH H 2 O Glucose H Maltose H H CH 2 OH O H OH H H CH 2 OH O H 1 glycosidic 4 OH Glucose H (b) CH 2 OH O H OH H Sucrose H HO H CH 2 OH

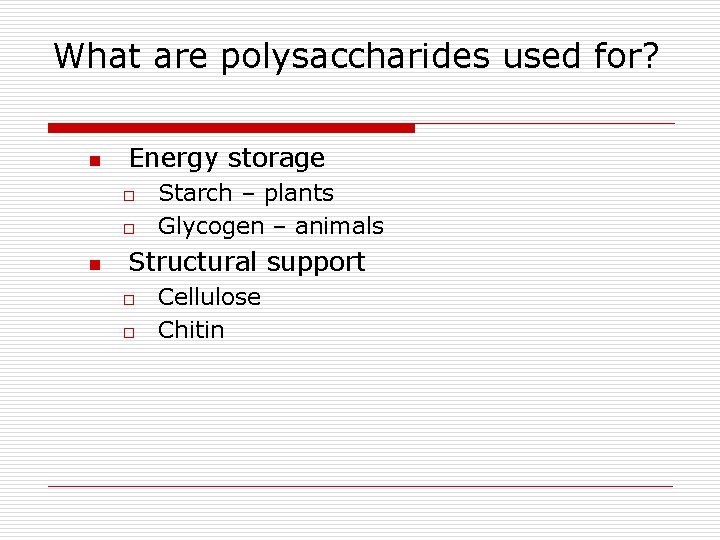
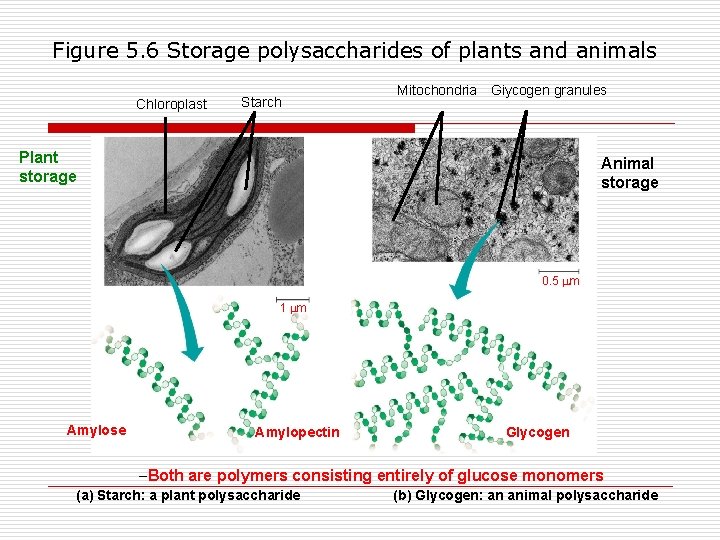
What are polysaccharides used for? n Energy storage o o n Starch – plants Glycogen – animals Structural support o o Cellulose Chitin

Figure 5. 6 Storage polysaccharides of plants and animals Chloroplast Starch Mitochondria Giycogen granules Plant storage Animal storage 0. 5 m 1 m Amylose Amylopectin Glycogen –Both are polymers consisting entirely of glucose monomers (a) Starch: a plant polysaccharide (b) Glycogen: an animal polysaccharide

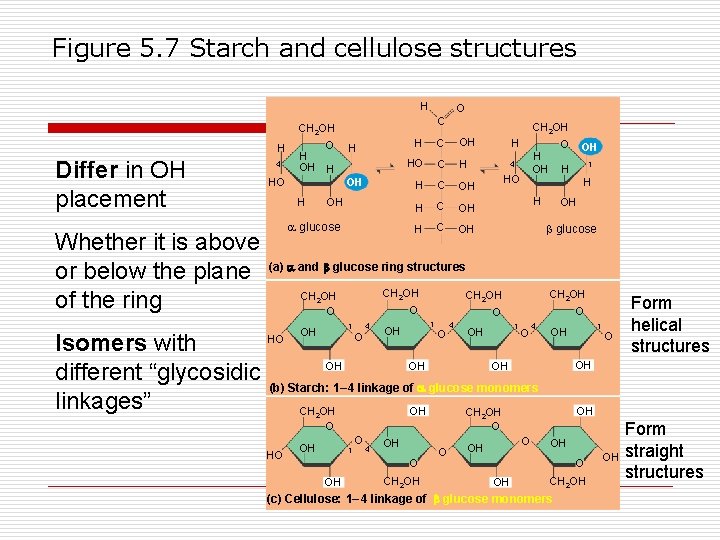
Figure 5. 7 Starch and cellulose structures H O C CH 2 OH H Differ in OH placement Whether it is above or below the plane of the ring Isomers with different “glycosidic linkages” 4 O H OH HO H OH glucose OH C H H CH 2 OH HO C H H C OH H H OH 4 HO H O OH H 1 H OH glucose (a) and glucose ring structures CH 2 OH O HO 4 1 OH O CH 2 OH O O O 1 OH O 4 OH OH 1 OH O 4 1 OH O Form helical structures OH OH (b) Starch: 1– 4 linkage of glucose monomers OH CH 2 OH O HO OH 1 O 4 OH O OH CH 2 OH O OH CH 2 OH (c) Cellulose: 1– 4 linkage of glucose monomers OH Form straight structures

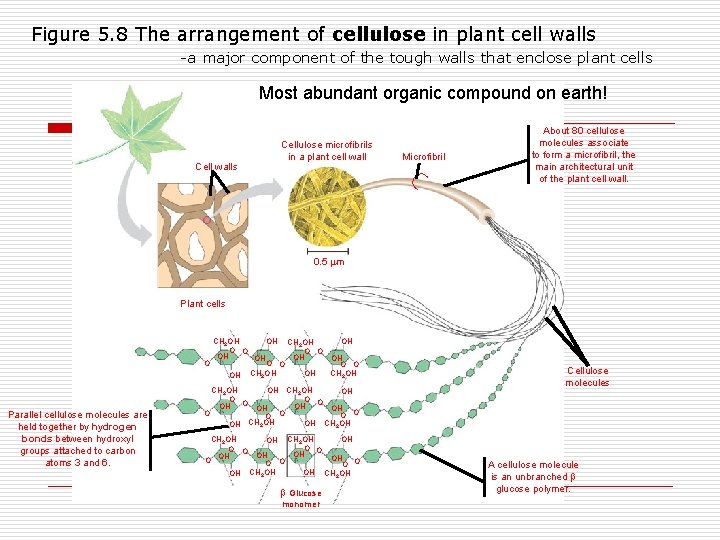
Figure 5. 8 The arrangement of cellulose in plant cell walls -a major component of the tough walls that enclose plant cells Most abundant organic compound on earth! Microfibril Cell walls Cellulose microfibrils in a plant cell wall About 80 cellulose molecules associate to form a microfibril, the main architectural unit of the plant cell wall. 0. 5 m Plant cells OH CH 2 OH O O OH OH O O O CH OH OH CH 2 OH 2 OH Parallel cellulose molecules are held together by hydrogen bonds between hydroxyl groups attached to carbon atoms 3 and 6. CH 2 OH OH O O OH CH 2 OH OH OH CH 2 OH O O OH OH OH O O O CH OH OH CH OH 2 2 OH Glucose monomer Cellulose molecules A cellulose molecule is an unbranched glucose polymer.

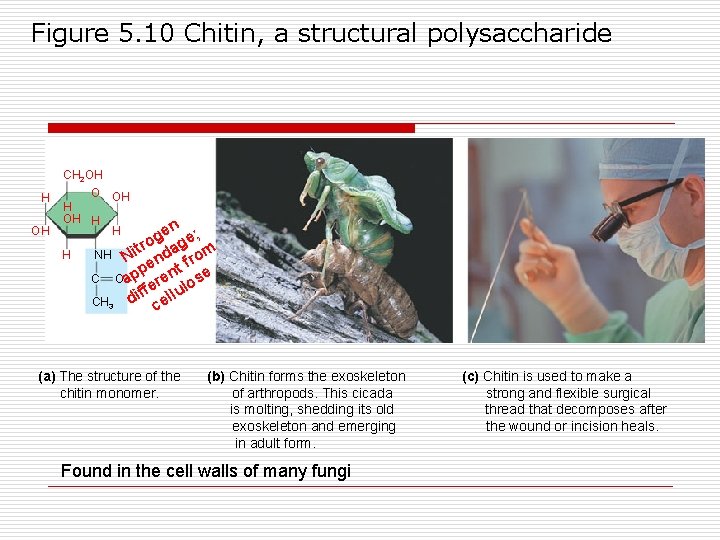
Figure 5. 10 Chitin, a structural polysaccharide H OH CH 2 OH O OH H H H n ge ge; o r NH Nit nda rom pe t f e C O ap ren e lulos f f i CH 3 d cel (a) The structure of the chitin monomer. (b) Chitin forms the exoskeleton of arthropods. This cicada is molting, shedding its old exoskeleton and emerging in adult form. Found in the cell walls of many fungi (c) Chitin is used to make a strong and flexible surgical thread that decomposes after the wound or incision heals.

Review Questions o o The building blocks of carbohydrates are? Function in? A glycosidic linkage is between what? What is the polysaccharide of plants called? Of animals? How does a cellulose molecule differ from a starch? Differ from a chitin?

Lipids Fats Oils Waxes Phospholipids Steroids Smallest unit – fatty acid + glycerol

Lipids o o o do not form polymers. little or no affinity for water. mostly of hydrocarbons form nonpolar covalent bonds. major function - energy storage.

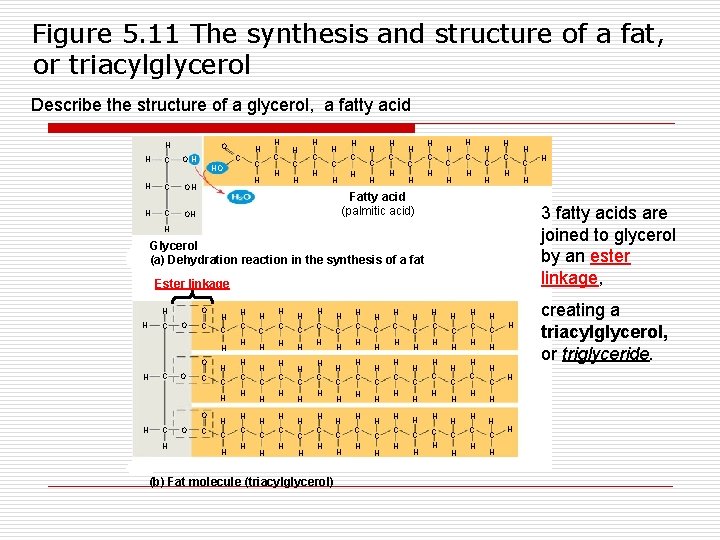
Figure 5. 11 The synthesis and structure of a fat, or triacylglycerol Describe the structure of a glycerol, a fatty acid H H C OH HO H C OH C H H H C C H H H C H H C H Fatty acid (palmitic acid) Glycerol (a) Dehydration reaction in the synthesis of a fat Ester linkage O H C O C H C H O C H H C H H C H H C H H C H (b) Fat molecule (triacylglycerol) H C H H C H H C H H C H H H 3 fatty acids are joined to glycerol by an ester linkage, H H H C H H C H H C H H H C H H creating a triacylglycerol, or triglyceride.

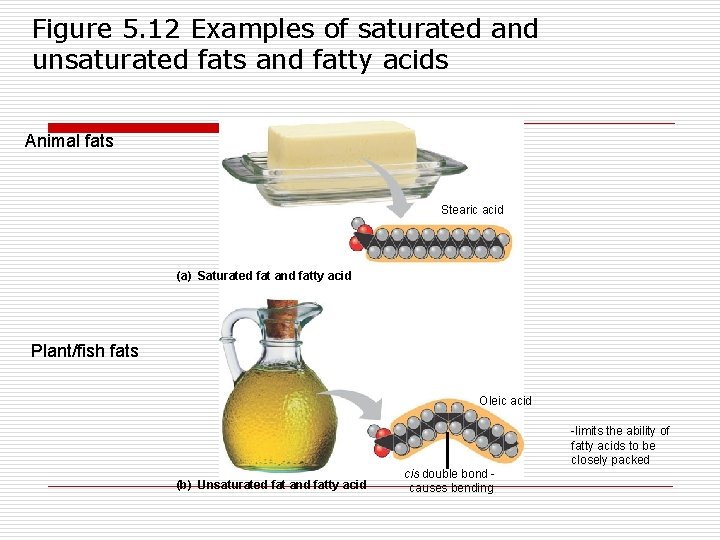
Figure 5. 12 Examples of saturated and unsaturated fats and fatty acids Animal fats Stearic acid (a) Saturated fat and fatty acid Plant/fish fats Oleic acid -limits the ability of fatty acids to be closely packed (b) Unsaturated fat and fatty acid cis double bond causes bending

Lipid Structure o o o Glycerol - a three-carbon alcohol with a hydroxyl group attached to each carbon. A fatty acid - a carboxyl group attached to a long carbon skeleton, often 16 to 18 carbons long. Hydrophobic due to many nonpolar C—H bonds in the long hydrocarbon skeleton

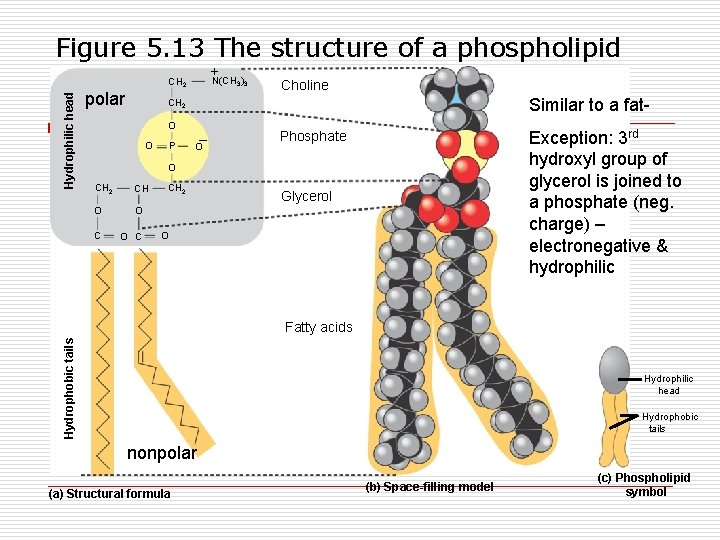
Figure 5. 13 The structure of a phospholipid +N(CH ) Hydrophilic head CH 2 polar 3 3 Choline Similar to a fat- CH 2 O O P – O Exception: 3 rd hydroxyl group of glycerol is joined to a phosphate (neg. charge) – electronegative & hydrophilic Phosphate O CH 2 CH O O C CH 2 Glycerol O Hydrophobic tails Fatty acids Hydrophilic head Hydrophobic tails nonpolar (a) Structural formula (b) Space-filling model (c) Phospholipid symbol

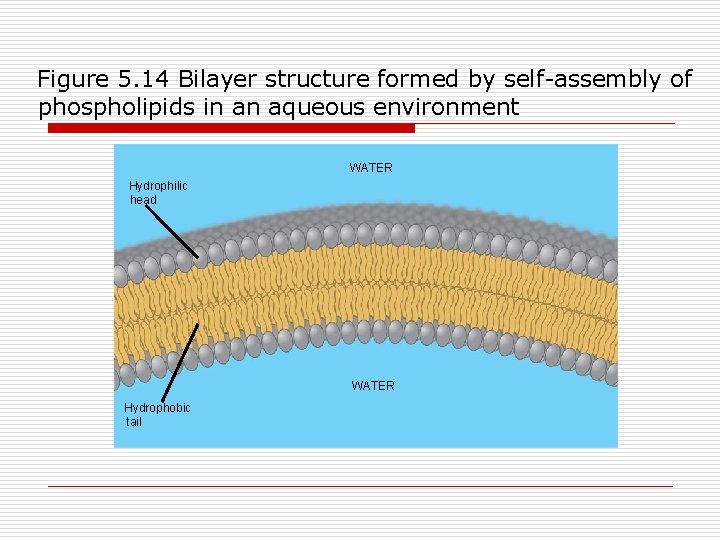
Figure 5. 14 Bilayer structure formed by self-assembly of phospholipids in an aqueous environment WATER Hydrophilic head WATER Hydrophobic tail

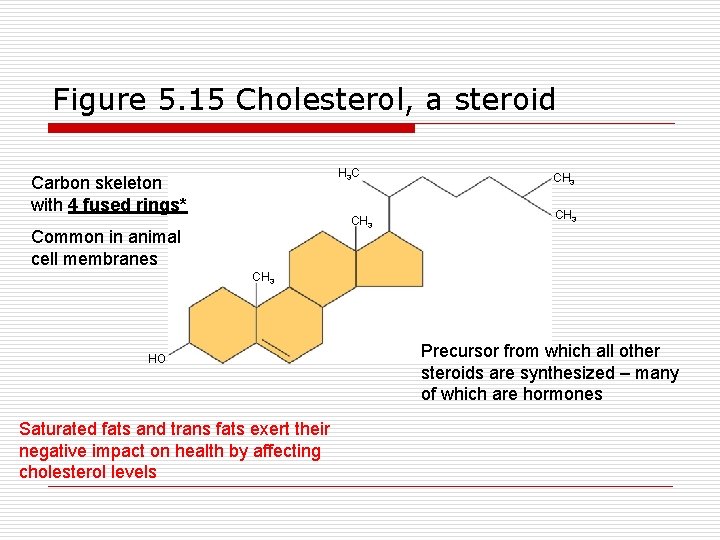
Figure 5. 15 Cholesterol, a steroid H 3 C Carbon skeleton with 4 fused rings* CH 3 Common in animal cell membranes CH 3 HO Saturated fats and trans fats exert their negative impact on health by affecting cholesterol levels Precursor from which all other steroids are synthesized – many of which are hormones

Questions - Lipids o o Common names for lipids? Smallest units? How are they different from the other 3 macromolecules? (bonding pattern, affinity for water, carbon chain, etc. ) An ester linkage is between?

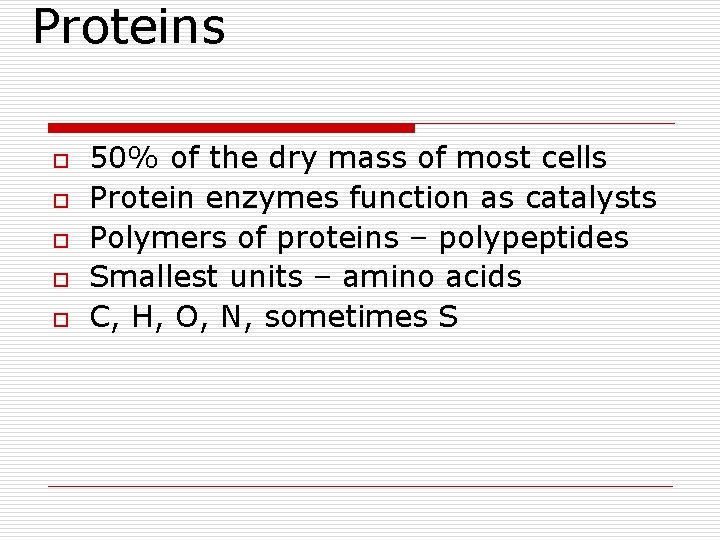
Proteins o o o 50% of the dry mass of most cells Protein enzymes function as catalysts Polymers of proteins – polypeptides Smallest units – amino acids C, H, O, N, sometimes S

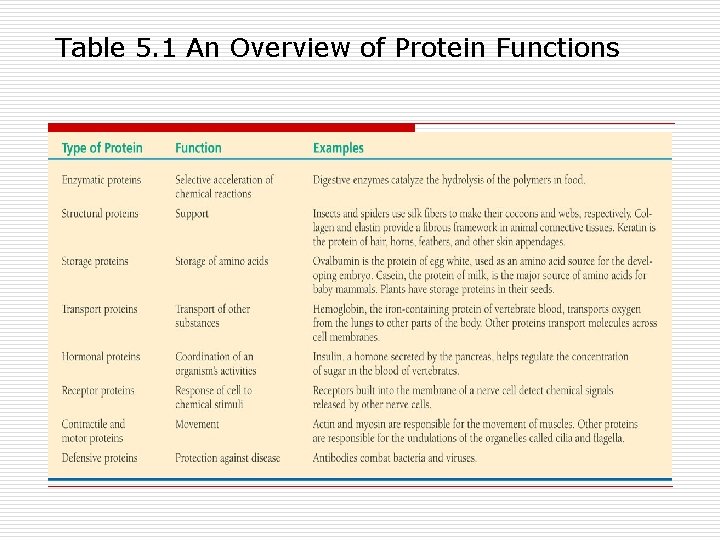
Table 5. 1 An Overview of Protein Functions

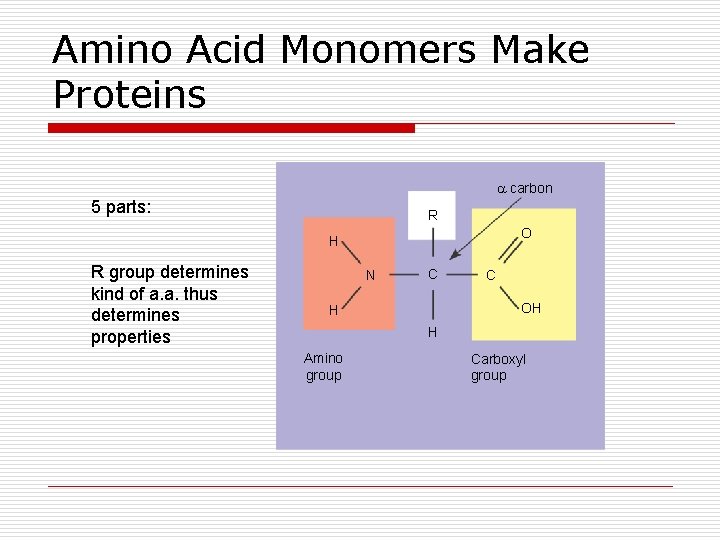
Amino Acid Monomers Make Proteins carbon 5 parts: R O H R group determines kind of a. a. thus determines properties N C C OH H H Amino group Carboxyl group

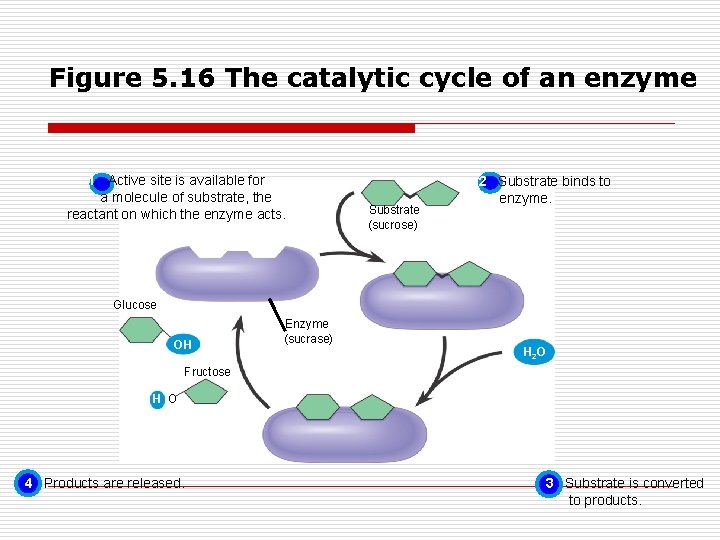
Figure 5. 16 The catalytic cycle of an enzyme 1 Active site is available for a molecule of substrate, the reactant on which the enzyme acts. Substrate (sucrose) 2 Substrate binds to enzyme. Glucose OH Enzyme (sucrase) H 2 O Fructose H O 4 Products are released. 3 Substrate is converted to products.

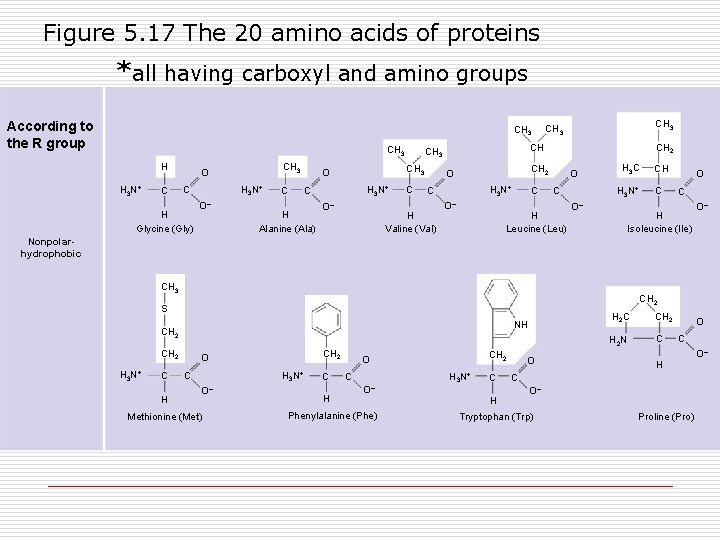
Figure 5. 17 The 20 amino acids of proteins *all having carboxyl and amino groups According to the R group CH 3 H H 3 N+ C CH 3 O H 3 N+ C H Glycine (Gly) O– C H 3 N+ C H Alanine (Ala) O– CH CH 3 O C CH 2 O H 3 N+ C H Valine (Val) CH 3 O– C O C H Leucine (Leu) H 3 C H 3 N+ O– CH C O C H Isoleucine (Ile) O– Nonpolarhydrophobic CH 3 CH 2 S NH CH 2 H 3 N+ C H CH 2 O H 3 N+ C O– Methionine (Met) C H CH 2 O C H 3 N+ O– Phenylalanine (Phe) C H O H 2 C CH 2 H 2 N C O C H C O– Tryptophan (Trp) Proline (Pro) O–

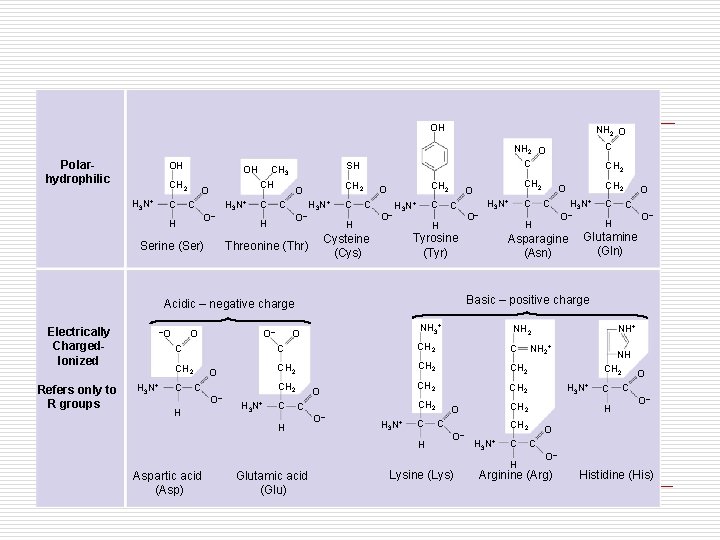
OH Polarhydrophilic OH CH 2 H 3 N+ C CH O H 3 N+ C O– H Serine (Ser) C CH 2 O H 3 N+ C O– H C CH 2 O C H O– C H 3 N+ O C O– H Refers only to R groups –O H 3 N+ O– O CH 2 C H 3 N+ O– C Asparagine (Asn) NH 3+ O CH 2 CH 2 C O– H H 3 N+ C O CH 2 C H O– H 3 N+ C H Aspartic acid (Asp) O C O– H Glutamine (Gln) NH 2 C C C H C O CH 2 Basic – positive charge Acidic – negative charge Electrically Charged. Ionized H 3 N+ Tyrosine (Tyr) Cysteine (Cys) Threonine (Thr) C NH 2 O C SH CH 3 OH NH 2 O Glutamic acid (Glu) C Lysine (Lys) NH 2+ H 3 N+ CH 2 O O– NH+ CH 2 H 3 N+ C H NH CH 2 O C C O– H O C O– Arginine (Arg) Histidine (His)

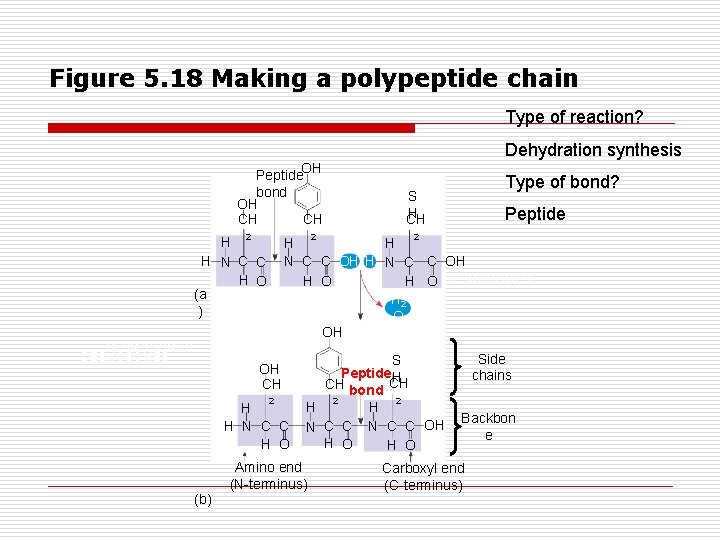
Figure 5. 18 Making a polypeptide chain Type of reaction? Dehydration synthesis Peptide. OH bond OH CH CH H (a ) DESMOSOMES N H 2 2 S H CH Peptide 2 H H N C C OH H O DESMOSOMES H 2 O OH C C H O OH CH 2 H H N C C H O (b) Type of bond? S Peptide. H CH bond CH 2 2 H H N C C OH H O Amino end (N-terminus) Side chains Backbon e Carboxyl end (C-terminus)

4 Levels of protein structure Primary --------Secondary--------------tertiary-------quaternary 1 +H 3 N Amino end Gly Pro Thr. Gly 5 Thr Gly Leu Met Val 15 Lya Val Leu Asp Glu Seu Pro Cya. Lya 10 pleated sheet Amino acid subunits R H C O C N H N H O C H C RHC R R N HO C O C N H C C R R H N N H 20 Ala Val Arg Gly Ser Pro 25 Ala O H H R R C C CH C C N C N CC N C C R R H H OH OH O O HH R R O C H H H H N N N C C C NH CN C H H O C C H ON H O C O R R H C O C C N H O H H C C N helix

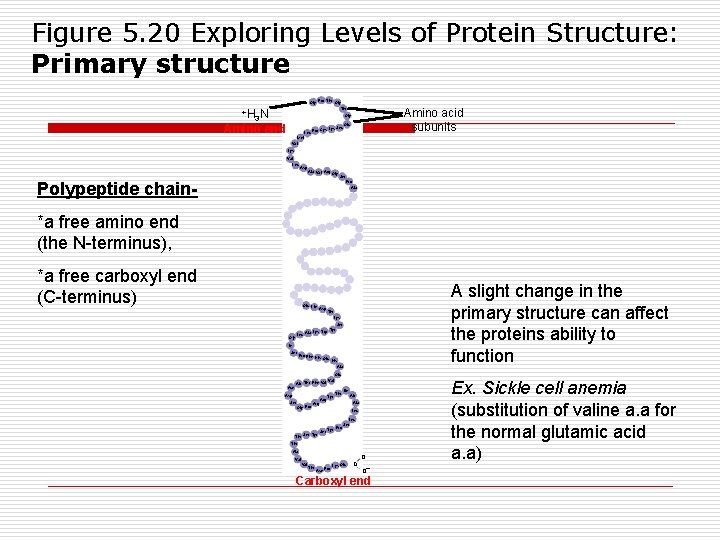
Figure 5. 20 Exploring Levels of Protein Structure: Primary structure Gly Pro Thr Gly Thr +H N 3 Amino acid subunits Gly Amino end Leu Seu Pro Cys Lys Glu Met Val Lys Val Leu Asp Ala Val Arg Gly Polypeptide chain- Ser Pro Ala *a free amino end (the N-terminus), *a free carboxyl end (C-terminus) Glu Lle Asp A slight change in the primary structure can affect the proteins ability to function Thr Lys Ser Lys Trp Tyr Leu Ala Gly lle Ser Pro Phe His Glu His Ala Glu Asn Ala Thr Phe Val Asp Ser Gly Pro Thr Arg Val Tyr Thr lle Ala Leu Ser Tyr Pro Leu Ser Thr Ala Val Thr Asn Pro Lys Glu c o o– Carboxyl end Ex. Sickle cell anemia (substitution of valine a. a for the normal glutamic acid a. a)

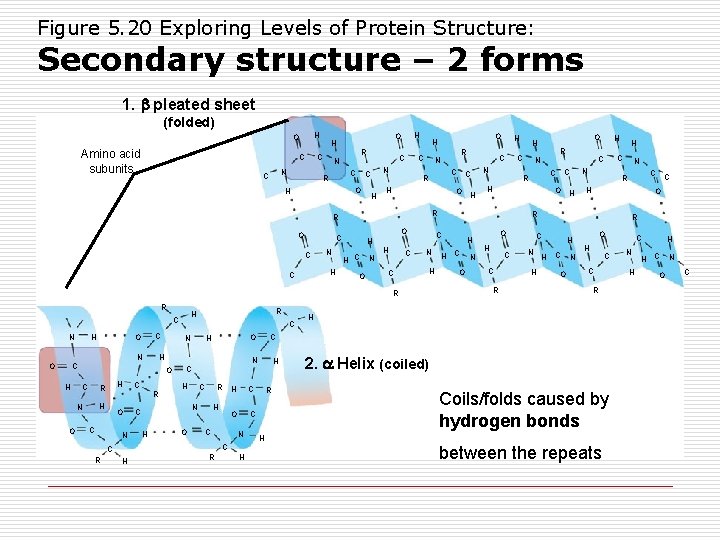
Figure 5. 20 Exploring Levels of Protein Structure: Secondary structure – 2 forms 1. pleated sheet (folded) H O Amino acid subunits C C N C R C H C R H C O C O H N C H H R O C N H C O N H C N C H C R N C H O H H C H R H C R C O N H H C R C N C C R H O H H N H C N C R H H H C O O H C N C R R C N H C O H H C O O H C N C R H C O H C C R H N O C C H N O R H C H N O H R C H R R C C O H R C N R O O H C N C O H H O H 2. Helix (coiled) Coils/folds caused by hydrogen bonds H between the repeats C N H H H C O N C

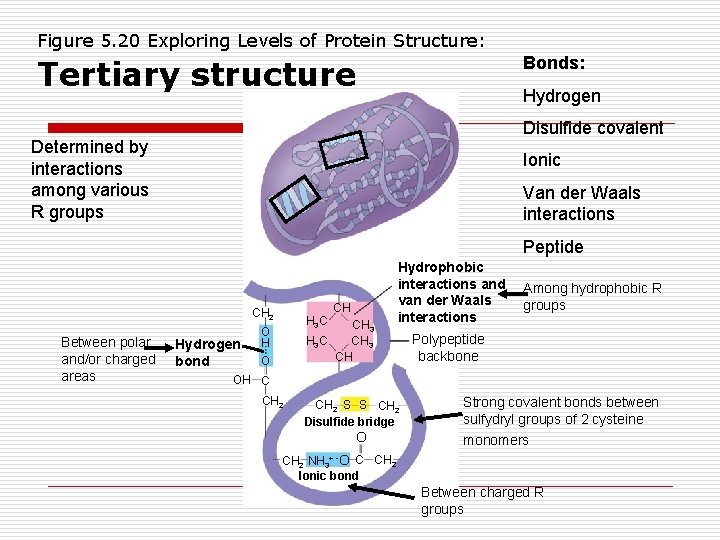
Figure 5. 20 Exploring Levels of Protein Structure: Bonds: Tertiary structure Hydrogen Disulfide covalent Determined by interactions among various R groups Ionic Van der Waals interactions Peptide CH 2 Between polar and/or charged areas Hydrogen bond H 3 C CH CH 3 H 3 C CH 3 CH O Hydrophobic interactions and van der Waals interactions Among hydrophobic R groups Polypeptide backbone OH C CH 2 S S CH 2 Disulfide bridge O Strong covalent bonds between sulfydryl groups of 2 cysteine monomers CH 2 NH 3+ -O C CH 2 Ionic bond Between charged R groups

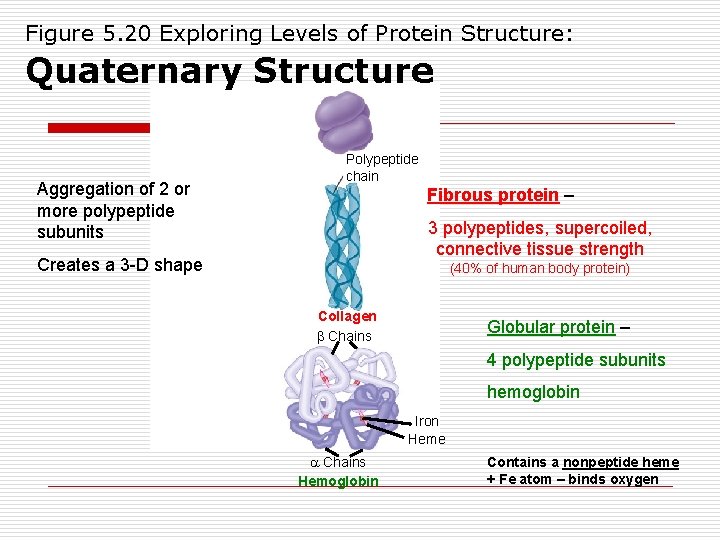
Figure 5. 20 Exploring Levels of Protein Structure: Quaternary Structure Aggregation of 2 or more polypeptide subunits Polypeptide chain Fibrous protein – 3 polypeptides, supercoiled, connective tissue strength Creates a 3 -D shape (40% of human body protein) Collagen Chains Globular protein – 4 polypeptide subunits hemoglobin Iron Heme Chains Hemoglobin Contains a nonpeptide heme + Fe atom – binds oxygen

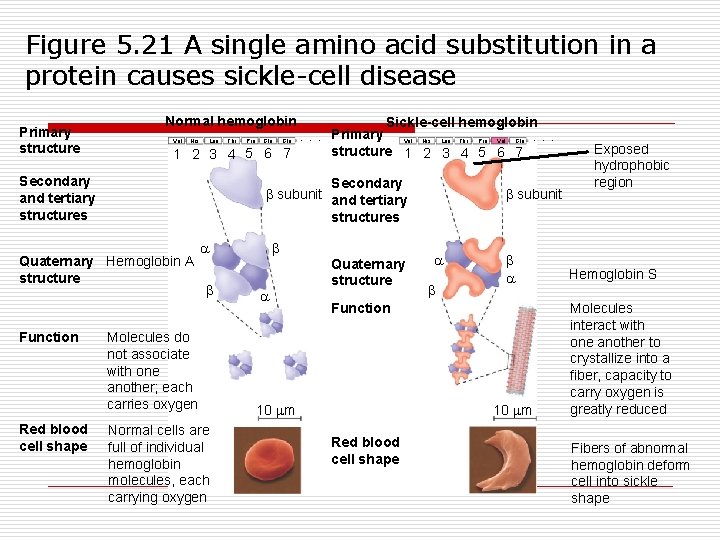
Figure 5. 21 A single amino acid substitution in a protein causes sickle-cell disease Primary structure Normal hemoglobin Sickle-cell hemoglobin Primary Val His Leu Thr Pro Glu. . . Val His Leu Thr Pro Val Glu. . . structure 1 2 3 4 5 6 7 Secondary and tertiary structures Secondary subunit and tertiary structures Quaternary Hemoglobin A structure Function Red blood cell shape Molecules do not associate with one another; each carries oxygen Normal cells are full of individual hemoglobin molecules, each carrying oxygen Quaternary structure subunit Function 10 m Red blood cell shape Exposed hydrophobic region Hemoglobin S Molecules interact with one another to crystallize into a fiber, capacity to carry oxygen is greatly reduced Fibers of abnormal hemoglobin deform cell into sickle shape

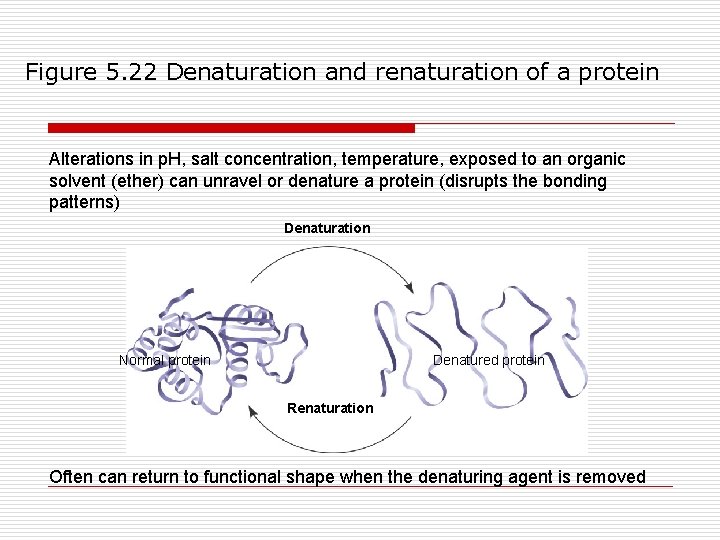
Figure 5. 22 Denaturation and renaturation of a protein Alterations in p. H, salt concentration, temperature, exposed to an organic solvent (ether) can unravel or denature a protein (disrupts the bonding patterns) Denaturation Normal protein Denatured protein Renaturation Often can return to functional shape when the denaturing agent is removed

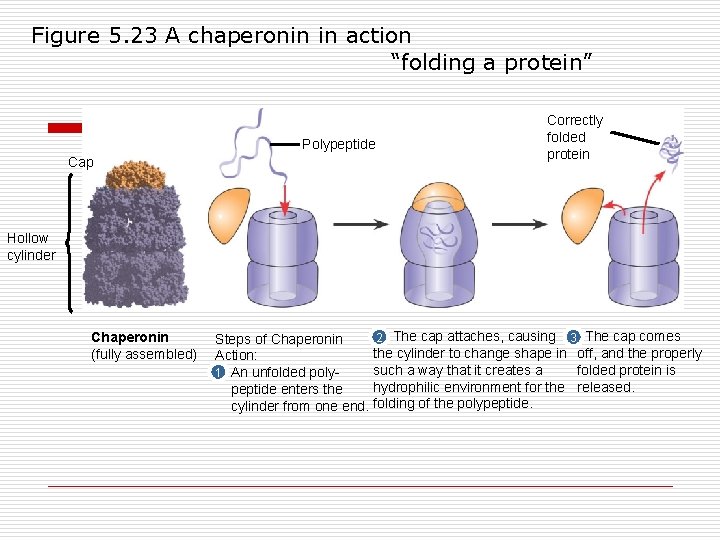
Figure 5. 23 A chaperonin in action “folding a protein” Polypeptide Cap Correctly folded protein Hollow cylinder Chaperonin (fully assembled) 2 The cap attaches, causing 3 The cap comes Steps of Chaperonin the cylinder to change shape in off, and the properly Action: such a way that it creates a folded protein is 1 An unfolded polyhydrophilic environment for the released. peptide enters the cylinder from one end. folding of the polypeptide.

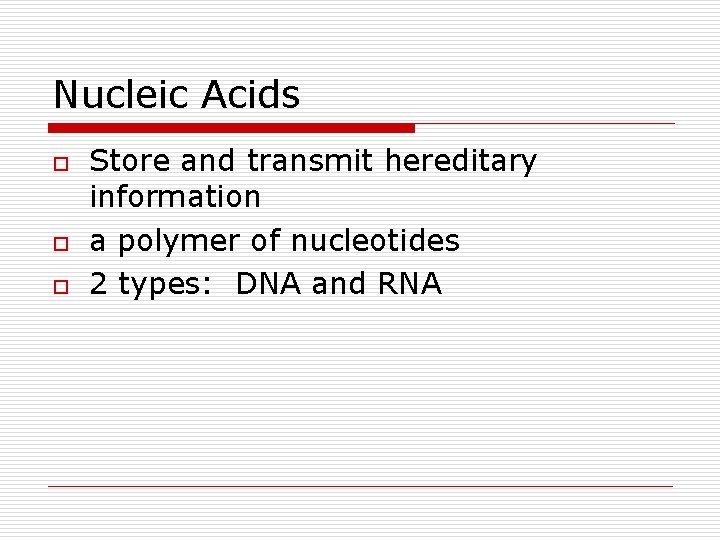
Nucleic Acids o o o Store and transmit hereditary information a polymer of nucleotides 2 types: DNA and RNA

Figure 5. 25 DNA RNA protein: a diagrammatic overview of information flow in a cell DNA 1 Synthesis of m. RNA in the nucleus m. RNA NUCLEUS CYTOPLASM m. RNA 2 Movement of m. RNA into cytoplasm via nuclear pore Ribosome 3 Synthesis of protein Polypeptide Amino acids

How does information from DNA make a protein? o o o DNA is copied to RNA in nucleus RNA travels to ribosome Amino acids brought to ribosome according to RNA code

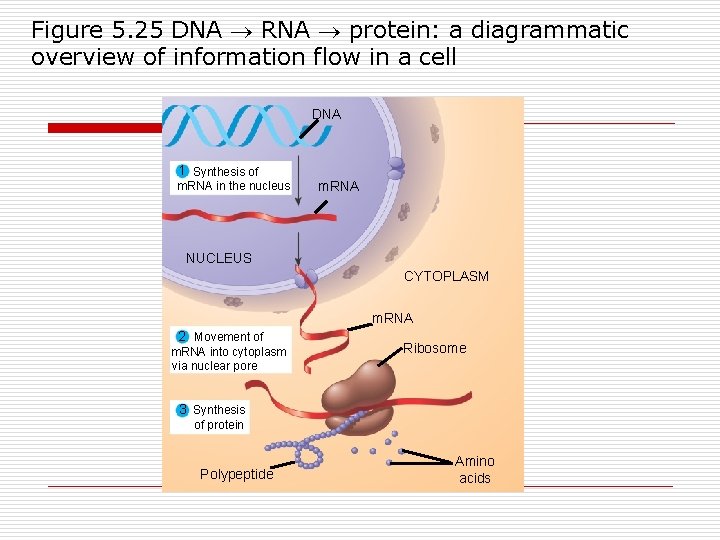
Figure 5. 26 The components of nucleic acids 5’ end (5 th carbon w/phosphate) 5’C O 3’C Nucleoside-2 parts O O 5’C O Nitrogenous base O 5’C CH 2 O O Phosphate 3’C Pentose group sugar 3’C (b) Nucleotide – 3 parts OH 3’ end carbon –OH attachment) (a) Polynucleotide, or nucleic acid Nitrogenous bases-rings of C, N Pyrimidines- single ring NH 2 O O C C CH 3 C N C CH HN HN CH CH C C CH N N O O H H H Cytosine. Thymine (in DNA)Uracil (in RNA) C U T Purines-double rings O NH 2 N C C N CC NH N HC HC CH N C N C NH 2 N H H Adenine Guanine A G (3 rd HOCH 2 O 4 Adjacent nucleotides are joined by covalent bonds called Phosphodiester linkages (formed between the –OH group on the 3’ and the phosphate on 5”) Pentose sugars 5’ OH H H 1’ 5’ HOCH 2 O OH 4 H H 1’ H 3’ 2’ H OH OH Deoxyribose (in DNA) Ribose (in RNA) (c) Nucleoside components

Smallest unit of a nucleic acid: o Nucleotide n n n Sugar Phosphate group Nitrogen base o o Adenine Guanine Cytosine Thymine

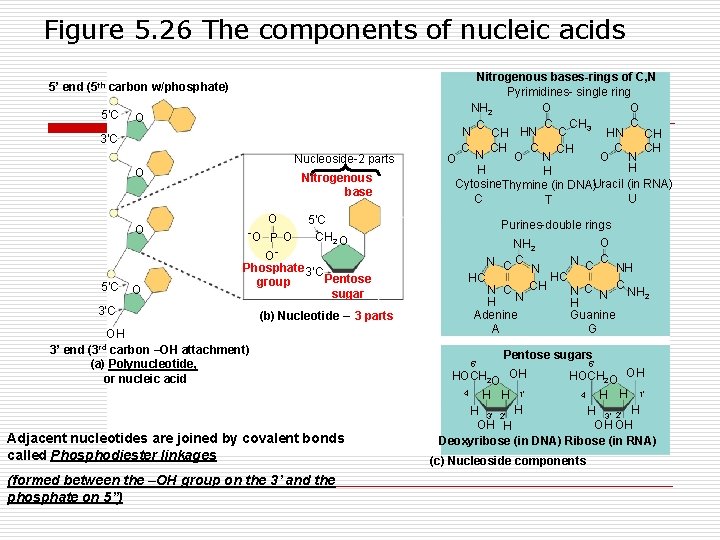
Figure 5. 27 The DNA double helix and its replication 3¢ end 5¢ end G C Sugar-phosphate backbone G C A T Hydrogen bonds C G A A Hold sides together T G Old strands C A A Base pair (joined by hydrogen bonding) T T Nucleotide about to be added to a new strand T A T C G A A 3¢ end G C T G C G G C T C A A G C T 5¢ end G A T C T A C 5¢ end A T G New strands A T C C G A T T C G A A T T A 3¢ end A T T G A 3¢ end 5¢ end