Ch 5 The Periodic Table Chapter 5 1

Ch. 5 The Periodic Table

Chapter 5. 1 Why do we need order? Makes it easier to find things We can understand a sequence We can make sense of a process

Who came up with the order of the Periodic Table? Dmitri Mendeleev – a Russian Chemist and teacher who was trying to compile a textbook for his students in 1860’s

What did he develop? Only had 63 elements in 1860’s Modeled his table after a card game (Solitaire) He made a card for each element Developed a pattern for the cards by putting them in order by increasing mass Then made sure each element in a row had similar properties

Why? - What was the reasoning behind Mendeleev’s order? Increasing mass Similar properties Helped him predict the existence of some elements (he left blanks in the table for them) Ex: eka-Aluminum = one below Al which would be a soft metal with a low melting point and D=5. 9 g/cm³ Gallium was discovered in 1875 (LED in some stoplights)

Why did Mendeleev get the credit? Mendeleev was not the 1 st to arrange the Periodic Table this way but he could explain how he did it & why he left blanks where he did. This order and reasoning allowed chemists to predict new elements, they knew what they were looking for and they could explain the chemical behaviors of different groups of elements.

Ch 5. 2 Periodic Table is sometimes compared to a piano because of its repetitive (periodic) pattern

Periodic Table Mendeleev’s Periodic Table had to be revised Mendeleev did not know that atoms of one element have the same # of protons Didn’t know that 2 different elements could not have the same # of protons

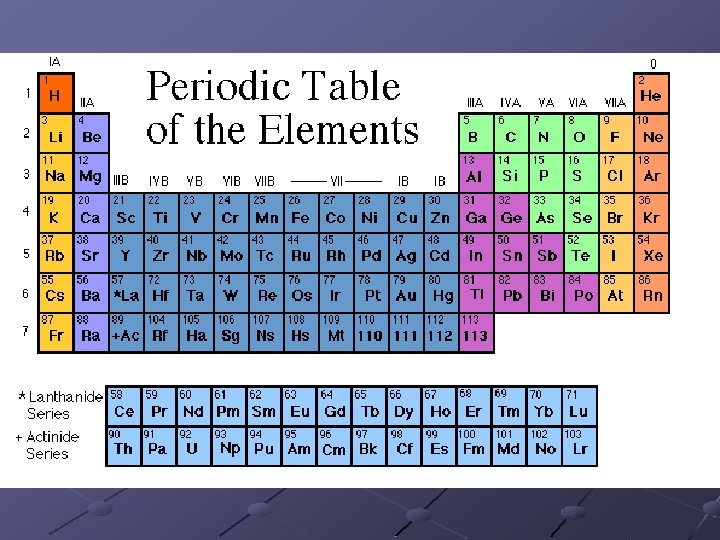
Periods Each row (side ways like dates on a calendar) is a Period # of elements in each period increases as you go down the table because the # of available orbitals increases from one energy level to the next How does the atomic # change in a period?

Groups Each column (like a support post or column on a porch) is a Group Elements in a Group have similar properties Groups have similar electron configurations (valence electrons) Electron Configuration determines an element’s chemical properties

Periodic Law This pattern of repeating properties is known as periodic law
Atomic Mass is a value that depends on the distribution of an element’s isotopes in nature and the mass of those isotopes. amu = atomic mass unit Isotope = have different # of neutrons In 1961, Scientists decided to use C-12 as the basis for amu 1 amu = 1/12 the atomic mass of C-12
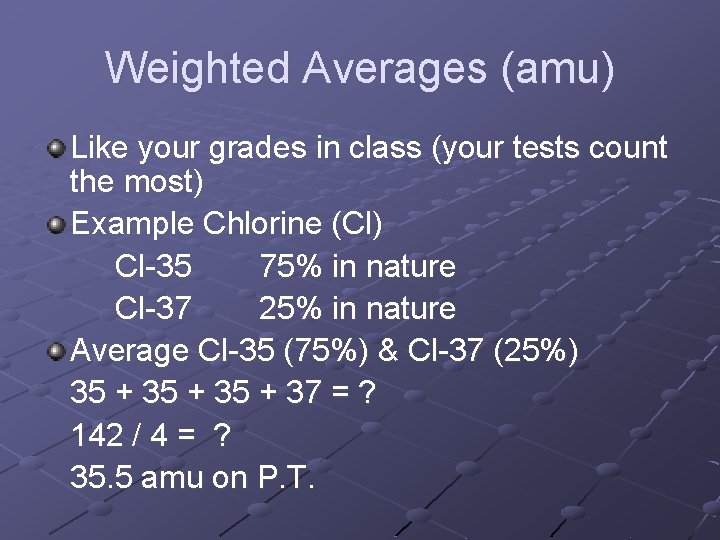
Weighted Averages (amu) Like your grades in class (your tests count the most) Example Chlorine (Cl) Cl-35 75% in nature Cl-37 25% in nature Average Cl-35 (75%) & Cl-37 (25%) 35 + 37 = ? 142 / 4 = ? 35. 5 amu on P. T.
Classification of Elements 1 st = by state of matter @ room temp. Solid - most metals and metalloids Liquid - Hg & Br Gas - H, N, O, F, Cl, & Noble Gases 2 nd = based on general properties Metals (Left side of P. T. ) Nonmetals (Right side of P. T. ) Metalloids (in between)

Metals Majority of elements in P. T. Good conductors of electricity & heat Solid @ room temp. (except ? ) Malleable Ductile

Transition Metals Some are very reactive & some are not (one evidence of reactivity = metals that change color – ex. ________) Transition Metals = Group 3 -12 (many form compounds with distinct colors – ex. Co can form Cobalt blue glass)

Nonmetals Poor conductors of heat & electricity Low boiling pt (most are gas @ room temp. ) All gases are nonmetals Any nonmetal that is a solid at room temp. tends to be brittle (will crumble or shatter if hit w/ a hammer) Vary from extremely reactive to not at all F is most reactive nonmetal Noble Gases are least reactive

Metalloids Properties b/t metals & nonmetals Metalloid’s ability to conduct electricity depends on temperature Ex. Si & Ge are good insulators @ low temps. but good conductors @ high temps.

Variation Across Periods Exception = Group 1 From L to R across a period, elements become less metallic and more nonmetallic in their properties Most reactive metals on L Most reactive nonmetals on R (except ? )
Valence Electrons An electron in the highest energy level of an atom # of valence electrons increases across a period from L to R Elements in a Group have similar properties b/c they have the same # of valence electrons H is a gas but is in group 1 A b/c it has only 1 valence electron

Alkali Metals Group 1 A Soft metals (Na = like cold butter) Only 1 valence electron Extremely reactive Found in nature only in compounds Reactivity of these elements increases from top to bottom (most reactive at bottom = Fr) Na & K are stored under oil to prevent reactions with H 2 O & O 2 Cs is stored in sealed glass tube w/ Ar gas

Alkaline Earth Metals Group 2 A 2 valence electrons Harder than metals in Group 1 A & have a higher melting point Mg = strong as steel but lighter weight (bicycles & back pack frames) Mg = in chlorophyll (helps make sugar in plants) Ca = in bones & teeth, part of compound that forms chalk, limestone, coral, pearls can be in toothpaste, a cast

Boron Family Group 3 A 3 val. electrons Al is the most abundant metal in Earth’s crust Al is strong, lightweight, malleable, & good conductor of electric current

Carbon Family Group 4 A 4 val. electrons C(nm), Si(mtld), Ge(mtld), Sn(mtl), Pb(mtl) Metallic properties increase from top to bottom of Group 4 A Carbon – except for H 2 O, most of the compounds in your body contain C

Nitrogen Family Group 5 A 5 val. electrons N(nm), P(nm), As(mtld), Sb(mtld), Bi(mtl) N & P = used for fertilizer White phosphorus will burst into flames when it contacts oxygen Red phosphorus is used to make matches ignite

Oxygen Family Group 6 A 6 val. electrons O(nm), Se(nm), Te(mtld), Po(mtld) O is the most abundant element in Earth’s crust Ozone is a form of O = O 3 S was 1 of 1 st elements discovered Molten Sulfur – used to make sulfuric acid (batteries), plastics, enamels, vulcanizing rubber, making dyes, & bleaching wood pulp Explosive, fatal if inhaled, can burn skin & be absorbed through skin, can be hard to see blue flames in daylight If tank, rail car, or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions http: //www. chemtradelogistics. com/MSDS/Molten_Sulfur-English. pdf

Halogens Group 7 A 7 val. electrons Highly reactive nonmetals (F is most reactive) F = in compound to prevent tooth decay, used to make nonstick pans Cl = bleach, kill bacteria in pools & drinking H 2 O I = keeps Thyroid working properly (from fish & salt) Br = Stable Br-79 exhibits a radioactive isomer, with a half-life of 4. 86 seconds. There at least 23 known isotopes of Br

Noble Gases Group 8 A He = 2 val. electrons Others = 8 val. Electrons Colorless, Odorless, Un-reactive Ar = used to store highly reactive elements All the Noble Gases except Rn are used in “neon” lights He =pink, Ne =orange-red, Ar =lavender, Kr =white, Xe =blue

The End Test on _____ Ch. 5
- Slides: 30