CH 4 Earth Chemistry Section 4 1 Matter















- Slides: 17

CH 4 Earth Chemistry

Section 4. 1 Matter is anything that takes up space and has mass. � An element is a substance that cannot be broken down into simpler, stable substances. � Most common elements in Earth’s crust: � › › Oxygen 46. 6% Silicon 27. 7% Aluminum 8. 1% Also Iron, Calcium, Sodium, Potassium, Magnesium, others

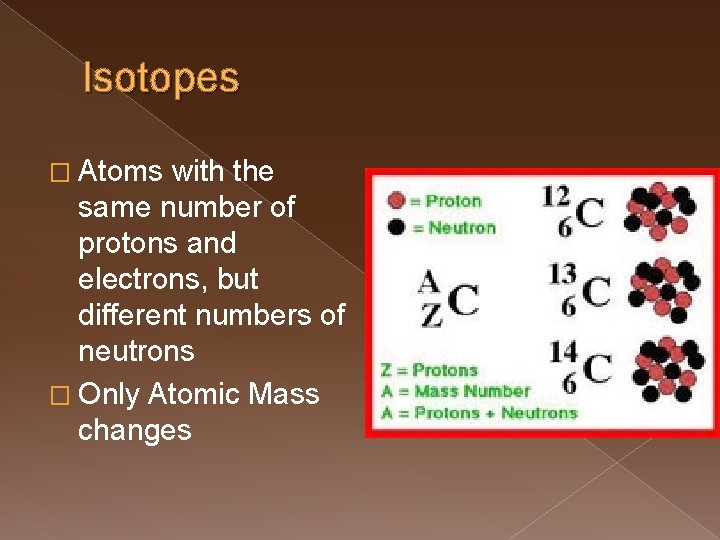
Isotopes � Atoms with the same number of protons and electrons, but different numbers of neutrons � Only Atomic Mass changes

Periodic Table � Go to Pg 86 � How is Atomic Mass Calculated? › Based on the isotopes that have different masses, the Periodic Table uses the average of the different masses for each element.

Practice Drawing Atoms � What does the atom of Oxygen look like? � What does the atom for Lithium look like? � What does the atom for Boron look like?

Why are the outer shell electrons important? � These are the electrons that are used for reactions! The outermost electrons are able to react with other atoms � Also Opposites attract � Let’s Identify the Valence Electrons for the groups of the Periodic Table

Ions � Charged Atoms-Allow the atoms of the elements to react � Examples……. � Na+ + Cl- Na. Cl � Al 3+ + O 2 - Al 2 O 3 � Opposites Attract!!!

Types of Bonds � Covalent Bonds-A bond that is formed by the attraction between atoms that share electrons. › Very strong Bond � Ionic Bonds- The attractive force between oppositely charged ions that results from the transfer of electrons from one atom to another › Very weak bond

Types of Bonds � Polar Covalent Bonds-In between an ionic and covalent bond- a Bond that does not equally share electrons and therefore creates a polar bond

Mixtures � Elements and compounds are generally mixed together. � A mixture is a combination of two or more substances that are not chemically combined. � Heterogeneous Mixture- mixture that is not uniformly distributed. � Homogeneous Mixture- mixture that is uniformly distributed. IE a solution of Salt water.

Chapter 5 Minerals � Know the four criteria for minerals. � Explain what a silicon-oxygen tetrahedron is and the different arrangements. � Know the seven properties of minerals that help us identify them and be able to explain how to find hardness.

Minerals � Examples › Rubies › Gold Nugget › Salt �A mineral is a natural, usually inorganic solid that has a characteristic chemical composition, an orderly internal structure, and a characteristic set of physical properties.

Minerals in Chihuahua

� http: //channel. nationalgeographic. com/episo de/Videos/08822_00

Characteristics of Minerals � Is it inorganic? › Not made out of previously living things � Does it occur naturally? › Things made in nature not a lab � Is it crystalline solid? › Has a regular repeating crystalline structure � Does it have a consistent chemical composition › A common chemical formula � Pg 103

Properties of a Mineral � Color � Streak � Luster � Cleavage and Fracture � Hardness � Crystal � Density Shape

Read pg 109 -112 � Create notes or an graphic organizer explaining in detail the 7 main properties of minerals. � For bonus you can take notes or continue your graphic organizer on the Special Properties of Minerals from page 113 -114