Ch 4 Atomic Theory Explains the formation of

















- Slides: 37

Ch. 4 Atomic Theory Explains the formation of compounds 4. 3 Chemical Equations Words to Know: *balanced chemical equation *chemical reaction *conservation of mass p. 202 215 *products *reactants *skeleton equation *symbolic equation

• A chemical change is the process by which one or more substances are changed into one or more new, different substances. – It has new properties Chemical changes occur when new substances are created. Copper reacting in nitric acid See pages 202 - 203

How do we know a Chemical Change has occurred? 1) Formation of a gas. 2) Formation of heat and light together. 3) Formation of a precipitate. 4) Color change. 5) Temperature change. https: //www. sophia. org/tutorials/indicators of a chemical change

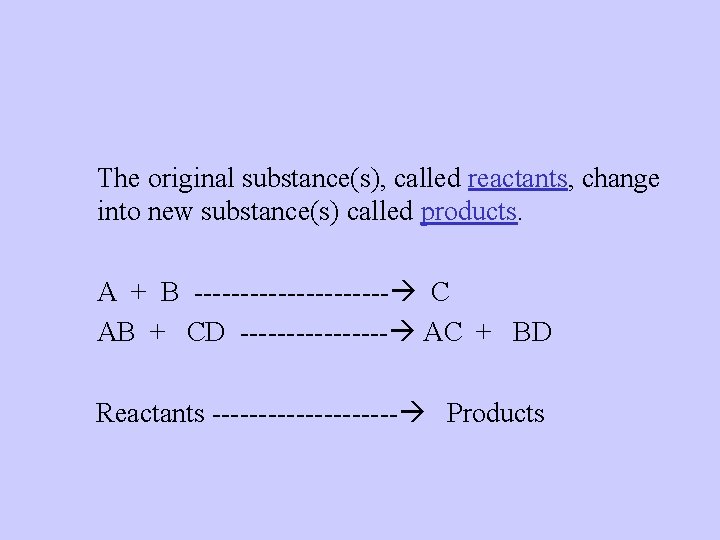
The original substance(s), called reactants, change into new substance(s) called products. A + B C AB + CD AC + BD Reactants Products

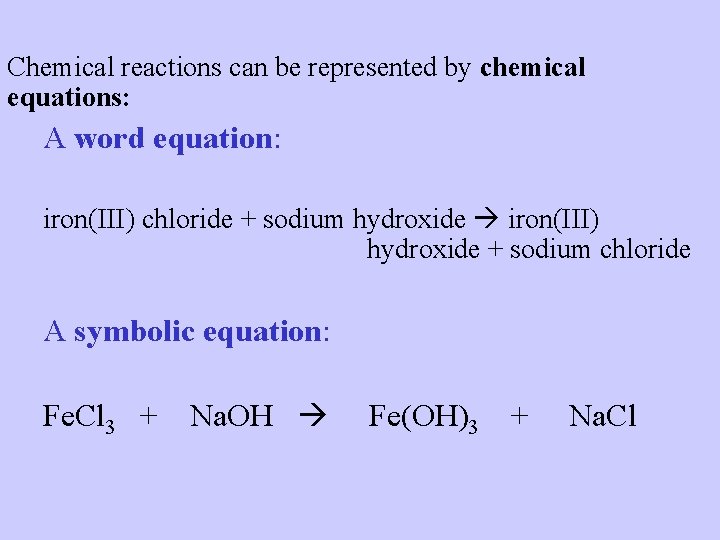
Chemical reactions can be represented by chemical equations: A word equation: iron(III) chloride + sodium hydroxide iron(III) hydroxide + sodium chloride A symbolic equation: Fe. Cl 3 + Na. OH Fe(OH)3 + Na. Cl

Both show: • reactants • products • a reaction arrow pointing from reactants to products • addition signs

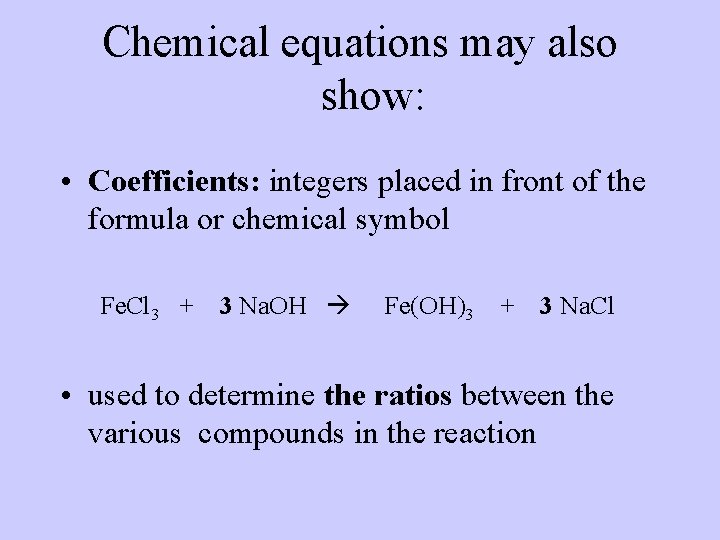
Chemical equations may also show: • Coefficients: integers placed in front of the formula or chemical symbol Fe. Cl 3 + 3 Na. OH Fe(OH)3 + 3 Na. Cl • used to determine the ratios between the various compounds in the reaction

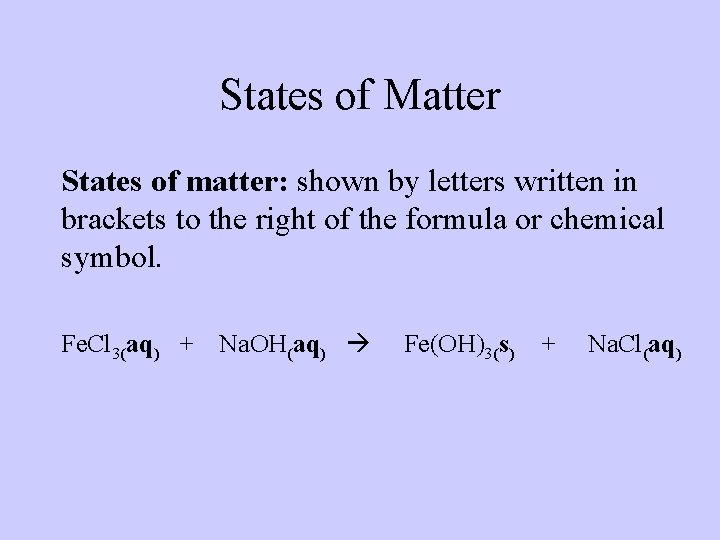
States of Matter States of matter: shown by letters written in brackets to the right of the formula or chemical symbol. Fe. Cl 3(aq) + Na. OH(aq) Fe(OH)3(s) + Na. Cl(aq)

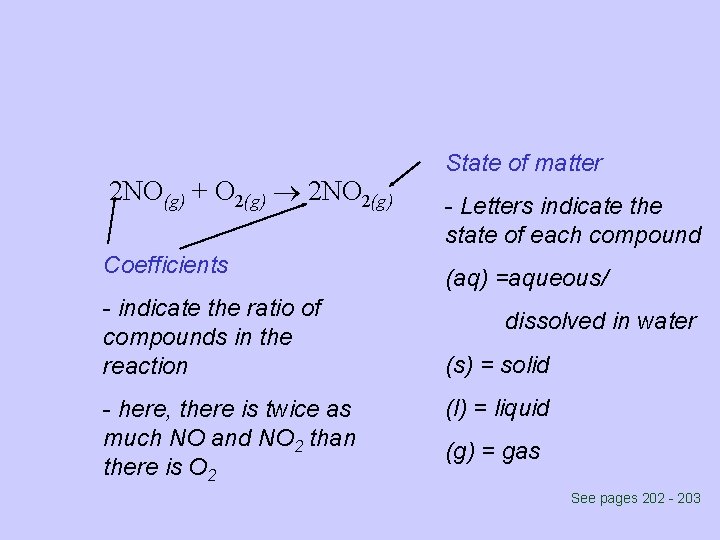
2 NO(g) + O 2(g) 2 NO 2(g) Coefficients - indicate the ratio of compounds in the reaction - here, there is twice as much NO and NO 2 than there is O 2 State of matter - Letters indicate the state of each compound (aq) =aqueous/ dissolved in water (s) = solid (l) = liquid (g) = gas See pages 202 - 203

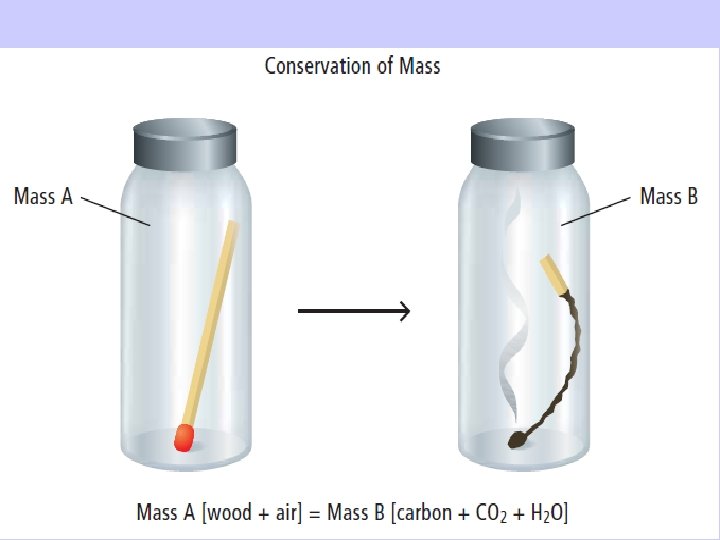
Conservation of Mass in Chemical Change Chemical change means new compounds are created. – BUT no new matter is created or destroyed; atoms are just rearranged. All matter in reactants = all matter in products See pages 204 - 205

Law of Conservation of Mass In 1785 French Chemist, Antoine Lavoisier and his wife Maire Anne stated, "We may lay it down as an incontestible axiom, that, in all the operations of art and nature, nothing is created; an equal quantity of matter exists both before and after the experiment; the quality and quantity of the elements remain precisely the same; and nothing takes place beyond changes and modifications in the combination of these elements. "

Law of Conservation of Mass: Matter can not be created nor can it be destroyed Total Mass of = Total Mass of Reactants Products

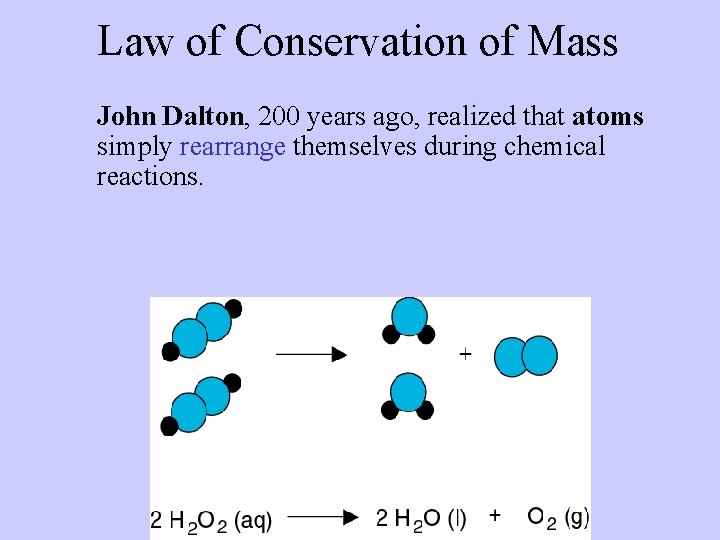
Law of Conservation of Mass John Dalton, 200 years ago, realized that atoms simply rearrange themselves during chemical reactions.

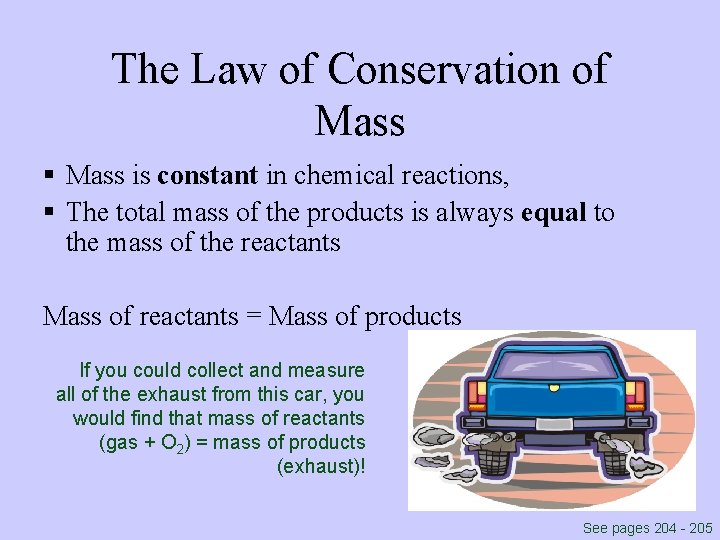
The Law of Conservation of Mass § Mass is constant in chemical reactions, § The total mass of the products is always equal to the mass of the reactants Mass of reactants = Mass of products If you could collect and measure all of the exhaust from this car, you would find that mass of reactants (gas + O 2) = mass of products (exhaust)! See pages 204 - 205


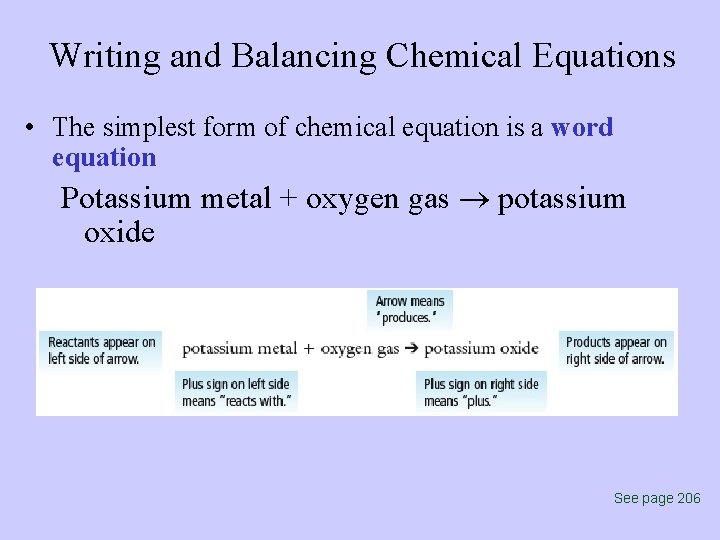
Writing and Balancing Chemical Equations • The simplest form of chemical equation is a word equation Potassium metal + oxygen gas potassium oxide See page 206

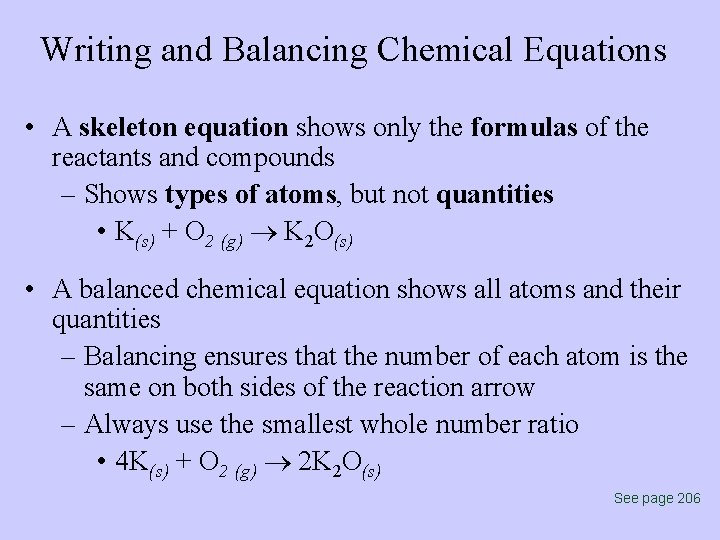
Writing and Balancing Chemical Equations • A skeleton equation shows only the formulas of the reactants and compounds – Shows types of atoms, but not quantities • K(s) + O 2 (g) K 2 O(s) • A balanced chemical equation shows all atoms and their quantities – Balancing ensures that the number of each atom is the same on both sides of the reaction arrow – Always use the smallest whole number ratio • 4 K(s) + O 2 (g) 2 K 2 O(s) See page 206

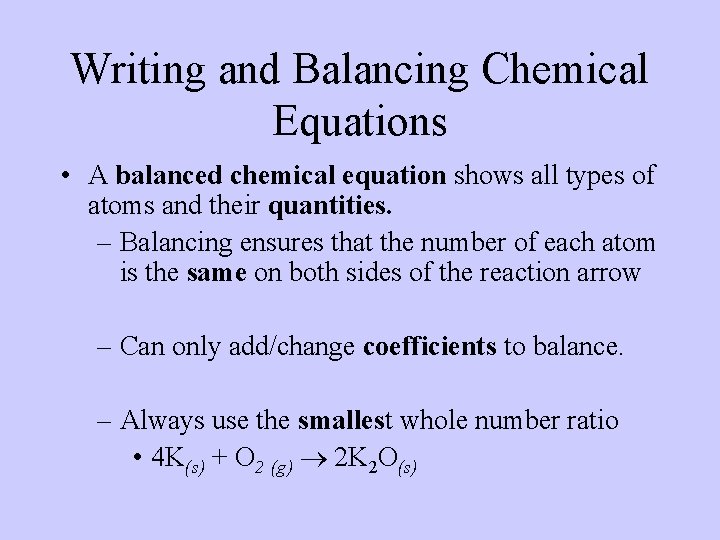
Writing and Balancing Chemical Equations • A balanced chemical equation shows all types of atoms and their quantities. – Balancing ensures that the number of each atom is the same on both sides of the reaction arrow – Can only add/change coefficients to balance. – Always use the smallest whole number ratio • 4 K(s) + O 2 (g) 2 K 2 O(s)

Counting Atoms We count atoms in the following way: Reactants: Na 2 CO 3 + Ca. Cl 2 Na 2 CO 3 means 1 molecule of Na 2 CO 3 ( 1 x 2 Na, 1 C, and 1 x 3 O) Ca. Cl 2 means 1 molecule of Ca. Cl 2 ( 1 x Ca and 1 x 2 Cl)

Counting Atoms Products: Ca. CO 3 + 2 Na. Cl Ca. CO 3 means 1 molecule of Ca. CO 3 ( 1 x 1 Ca, 1 C, and 1 x 3 O) 2 Na. Cl means 2 molecules of Na. Cl ( 2 x 1 Na and 2 x 1 Cl)

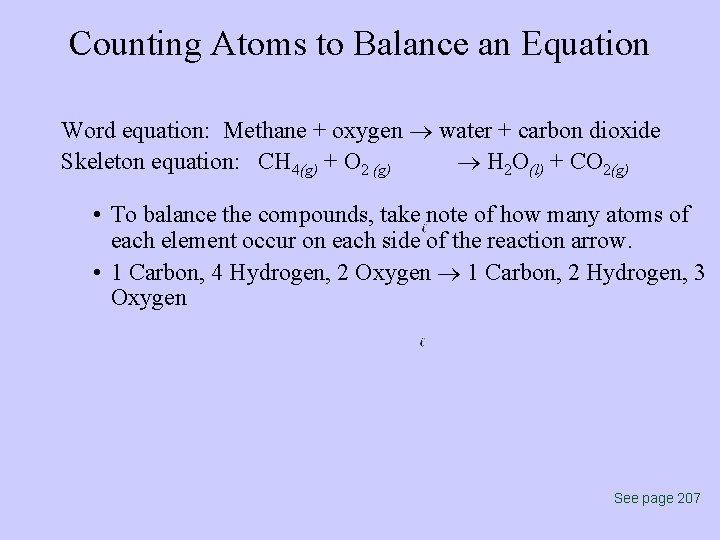
Counting Atoms to Balance an Equation Word equation: Methane + oxygen water + carbon dioxide Skeleton equation: CH 4(g) + O 2 (g) H 2 O(l) + CO 2(g) • To balance the compounds, take note of how many atoms of each element occur on each side of the reaction arrow. • 1 Carbon, 4 Hydrogen, 2 Oxygen 1 Carbon, 2 Hydrogen, 3 Oxygen See page 207

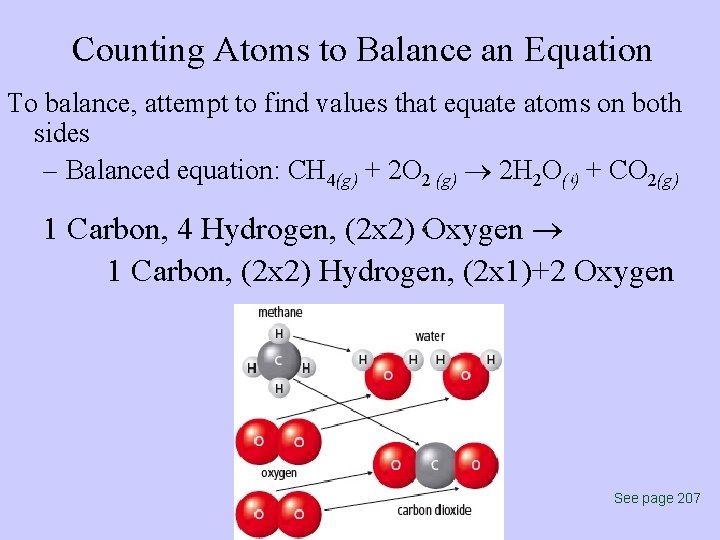
Counting Atoms to Balance an Equation To balance, attempt to find values that equate atoms on both sides – Balanced equation: CH 4(g) + 2 O 2 (g) 2 H 2 O( ) + CO 2(g) 1 Carbon, 4 Hydrogen, (2 x 2) Oxygen 1 Carbon, (2 x 2) Hydrogen, (2 x 1)+2 Oxygen See page 207

Ex. Zinc metal and hydrochloric acid react to form zinc chloride and hydrogen gas word equation: zinc + hydrochloric acid zinc chloride + hydrogen gas

Try to write the following chemical reaction as a word equation Aluminium metal and copper(II) chloride solution react to form copper metal and aluminium chloride.


Copper(II) chloride solution reacts with sodium hydroxide to form copper(II) hydroxide and sodium chloride

Hints for Writing Word Equations • Chemical equations can be written using chemical names instead of formulas. Eg. The chemical reaction of photosynthesis in plants consumes light energy and covnerts it into chemical energy in the form of sugar. Word equation: carbon dioxide + water glucose + oxygen

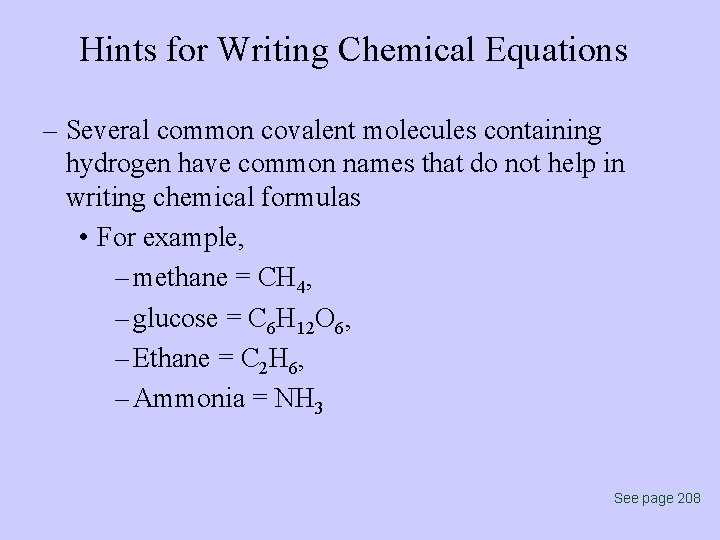
Hints for Writing Chemical Equations – Several common covalent molecules containing hydrogen have common names that do not help in writing chemical formulas • For example, – methane = CH 4, – glucose = C 6 H 12 O 6, – Ethane = C 2 H 6, – Ammonia = NH 3 See page 208

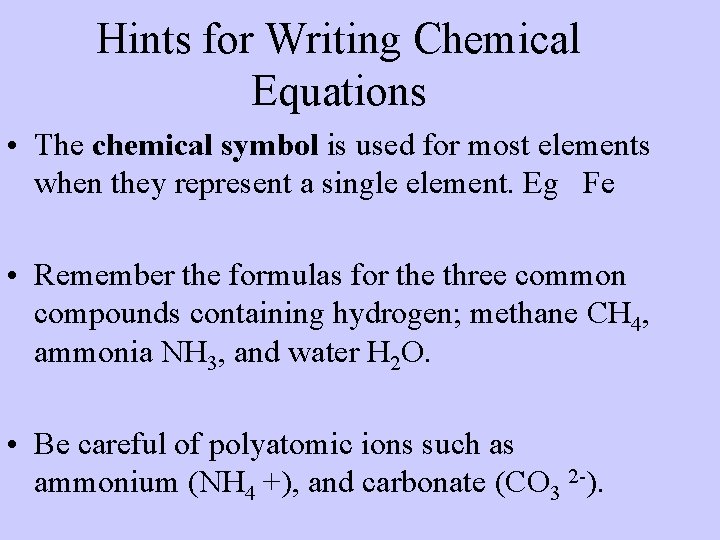
Hints for Writing Chemical Equations • The chemical symbol is used for most elements when they represent a single element. Eg Fe • Remember the formulas for the three common compounds containing hydrogen; methane CH 4, ammonia NH 3, and water H 2 O. • Be careful of polyatomic ions such as ammonium (NH 4 +), and carbonate (CO 3 2 ).

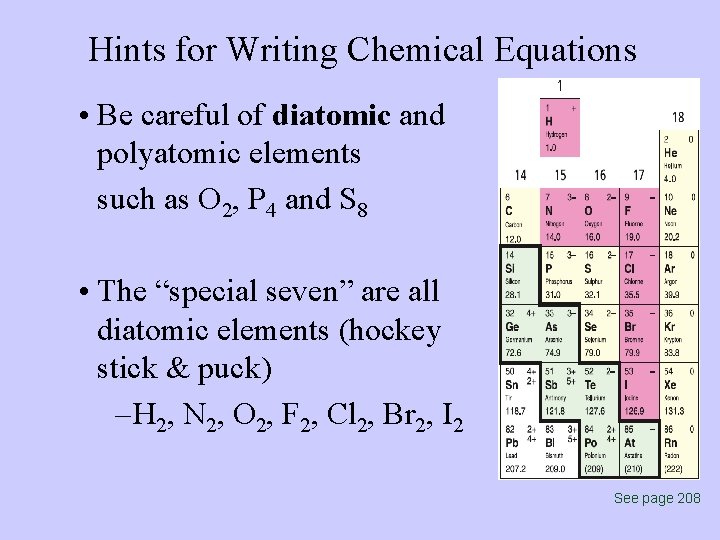
Hints for Writing Chemical Equations • Be careful of diatomic and polyatomic elements such as O 2, P 4 and S 8 • The “special seven” are all diatomic elements (hockey stick & puck) – H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2 See page 208

word equation: zinc + hydrochloric acid zinc chloride + hydrogen gas

• Try to write the following word equation as a skeleton equation: • aluminium + copper(II) chloride solution copper + aluminium chloride

• copper(II) chloride + sodium hydroxide copper(II) hydroxide + sodium chloride

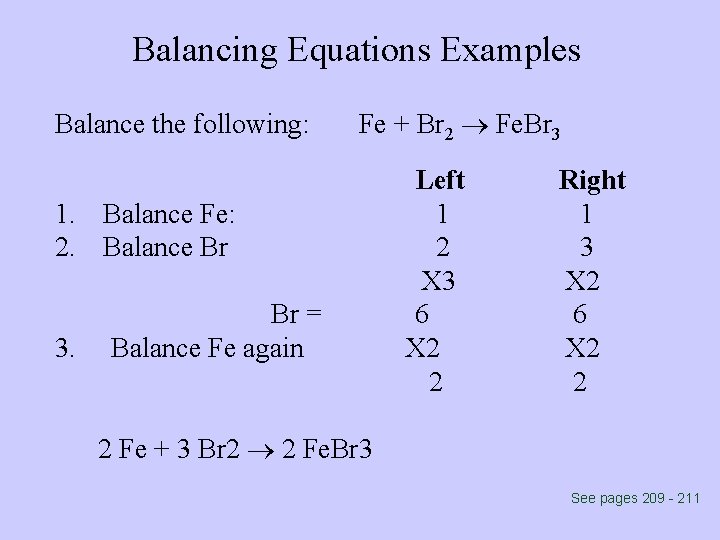
Balancing Equations Examples Balance the following: Fe + Br 2 Fe. Br 3 1. Balance Fe: 2. Balance Br 3. Br = Balance Fe again Left 1 2 X 3 6 X 2 2 Right 1 3 X 2 6 X 2 2 2 Fe + 3 Br 2 2 Fe. Br 3 See pages 209 - 211

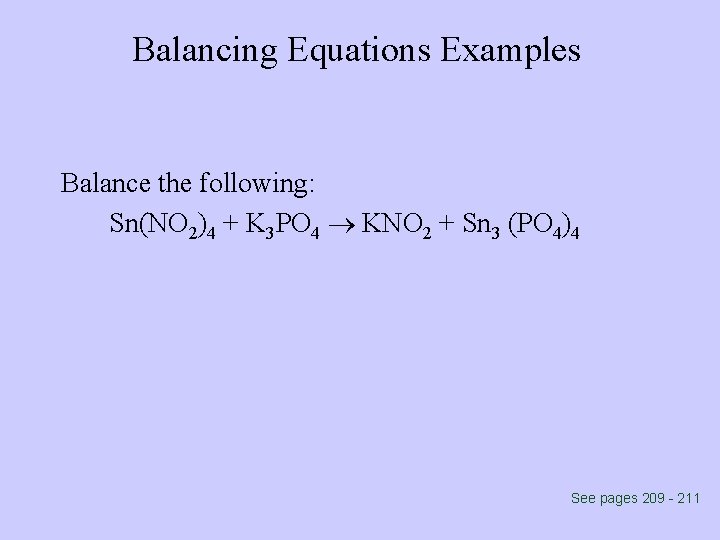
Balancing Equations Examples Balance the following: Sn(NO 2)4 + K 3 PO 4 KNO 2 + Sn 3 (PO 4)4 See pages 209 - 211

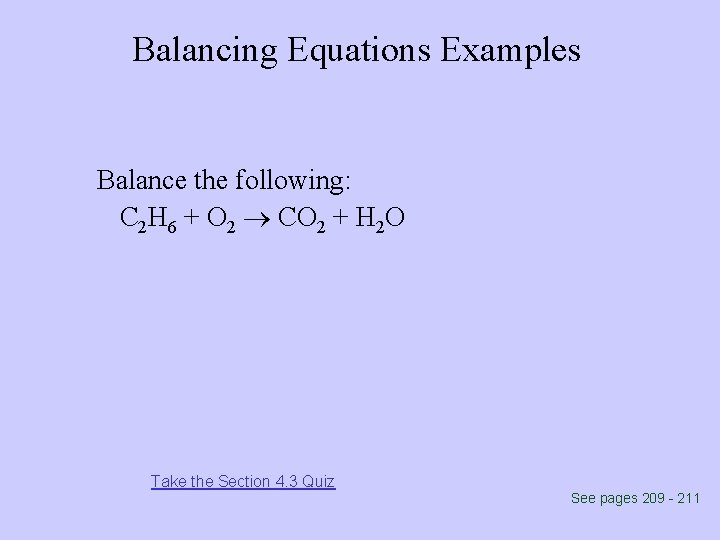
Balancing Equations Examples Balance the following: C 2 H 6 + O 2 CO 2 + H 2 O Take the Section 4. 3 Quiz See pages 209 - 211

Strategies for Balancing Equations – Trial and error will work, but can be very inefficient – Balance compounds first, single elements last – Balance one compound at a time – Only add coefficients; NEVER change subscripts! – Balance metals first, then nonmetals, then H & O – If H and O appear in more than one place, attempt to balance them LAST – Polyatomic ions (such as SO 42–) can often be balanced as a whole group – Always double-check after you think you are finished! See pages 209 - 211