Ch 4 Atomic Structure I Structure of the
- Slides: 15

Ch. 4 - Atomic Structure I. Structure of the Atom ¨ Dalton’s Atomic Theory ¨ Subatomic Particles

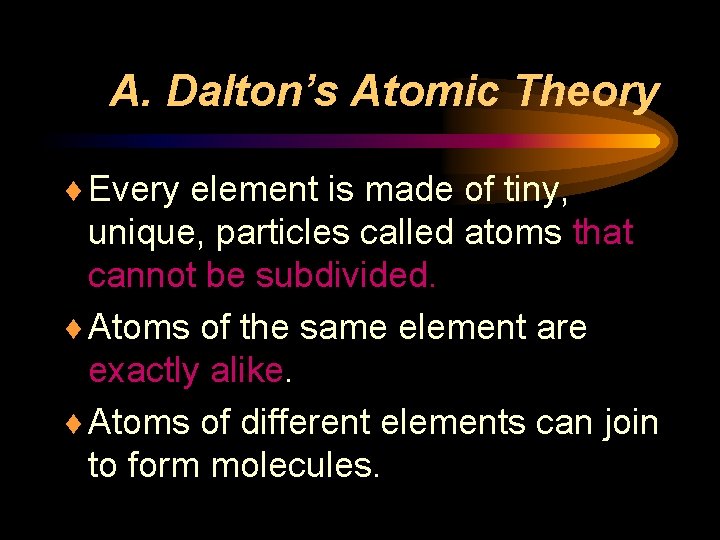
A. Dalton’s Atomic Theory ¨ Every element is made of tiny, unique, particles called atoms that cannot be subdivided. ¨ Atoms of the same element are exactly alike. ¨ Atoms of different elements can join to form molecules.

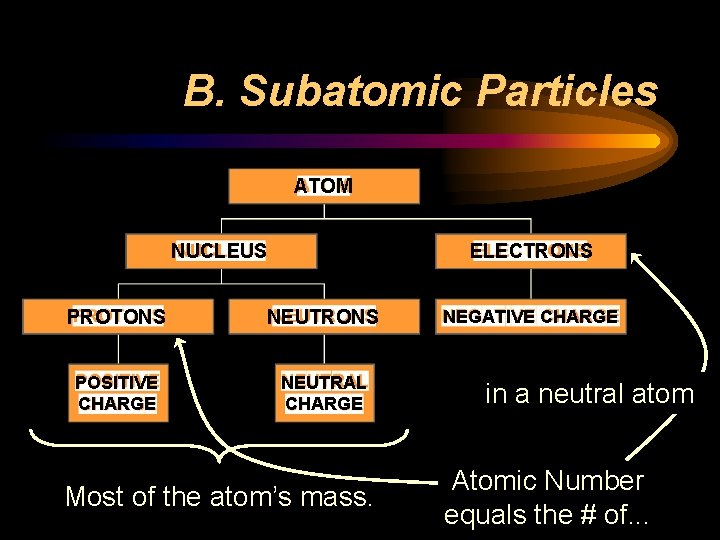
B. Subatomic Particles ATOM NUCLEUS ELECTRONS PROTONS NEUTRONS POSITIVE CHARGE NEUTRAL CHARGE Most of the atom’s mass. NEGATIVE CHARGE in a neutral atom Atomic Number equals the # of. . .

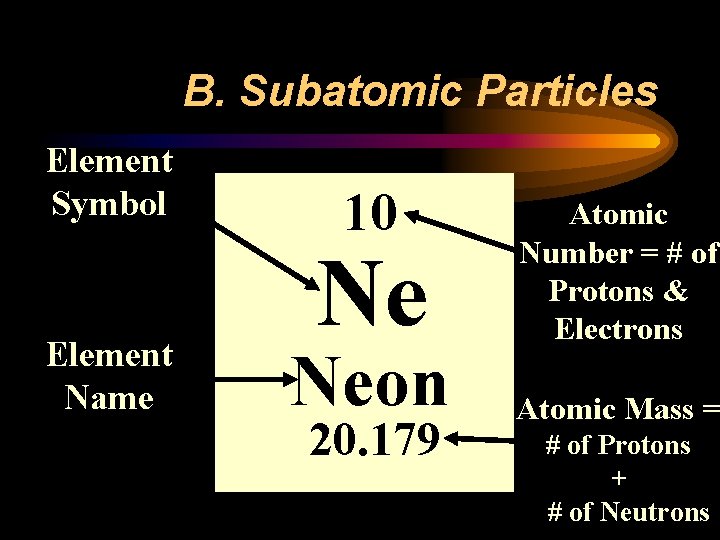
B. Subatomic Particles Element Symbol Element Name 10 Ne Neon 20. 179 Atomic Number = # of Protons & Electrons Atomic Mass = # of Protons + # of Neutrons

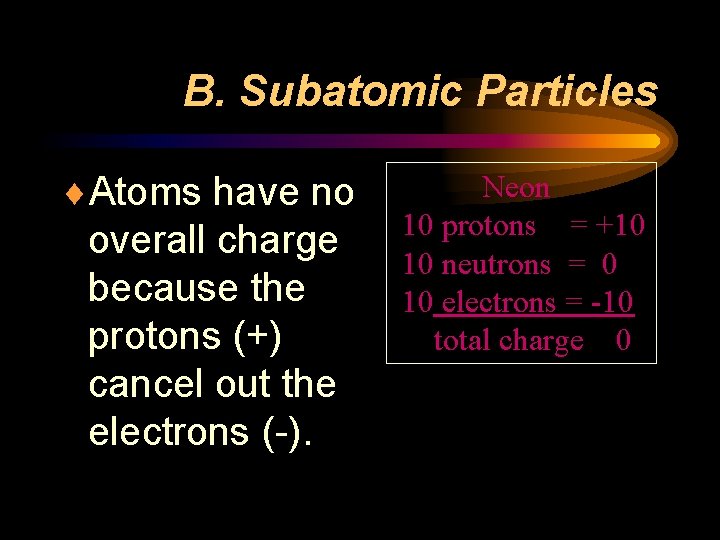
B. Subatomic Particles ¨Atoms have no overall charge because the protons (+) cancel out the electrons (-). Neon 10 protons = +10 10 neutrons = 0 10 electrons = -10 total charge 0

Ch. 4 - Atomic Structure II. Electron Cloud Model ¨ Orbital ¨ Energy Levels ¨ Bohr Model Diagrams

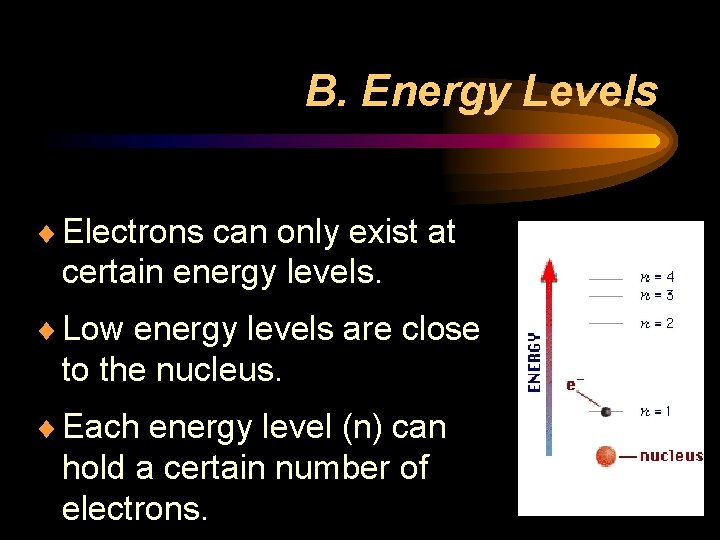
B. Energy Levels ¨ Electrons can only exist at certain energy levels. ¨ Low energy levels are close to the nucleus. ¨ Each energy level (n) can hold a certain number of electrons.

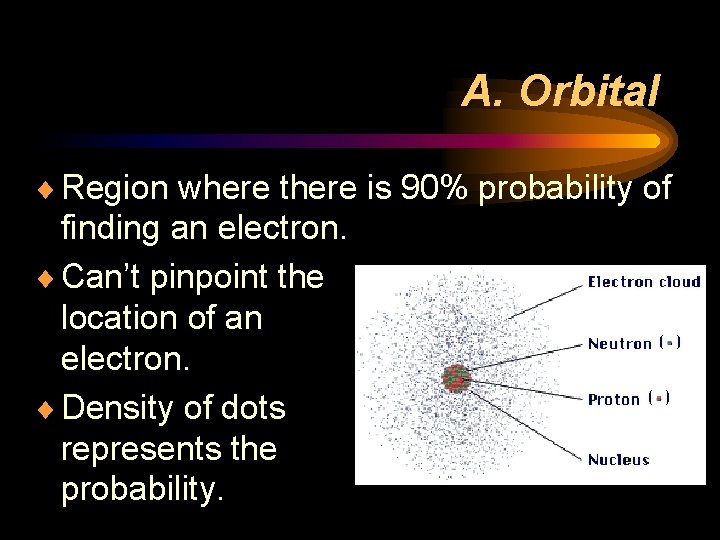
A. Orbital ¨ Region where there is 90% probability of finding an electron. ¨ Can’t pinpoint the location of an electron. ¨ Density of dots represents the probability.

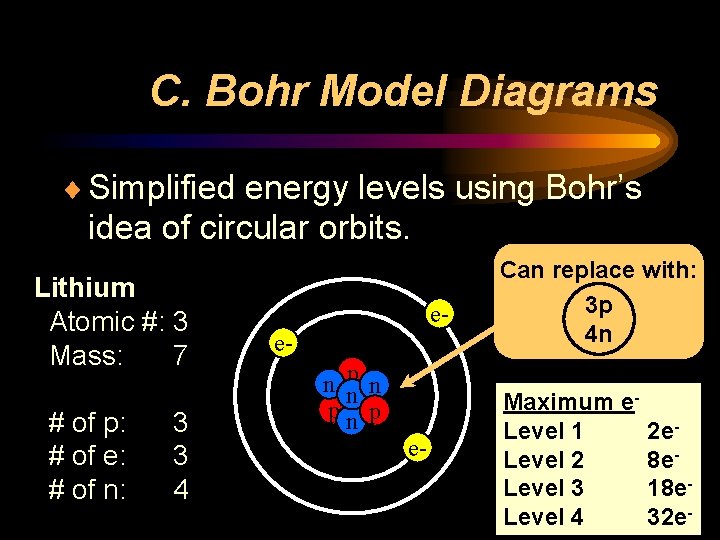
C. Bohr Model Diagrams ¨ Simplified energy levels using Bohr’s idea of circular orbits. Lithium Atomic #: 3 Mass: 7 # of p: # of e: # of n: 3 3 4 een np n pn p e- Can replace with: 3 p 4 n Maximum e. Level 1 Level 2 Level 3 Level 4 2 e 8 e 18 e 32 e-

C. Bohr Model Activity ¨ Choose a number between 1 & 18. ¨ Find your element by the atomic number you picked. ¨ Draw a Bohr Model diagram for your element on your marker board. • Round off the atomic mass listed on the table and subtract the atomic # to find the # of neutrons. • Abbreviate the # of ‘p’ and ‘n’ in the nucleus. ¨ Have a partner check your drawing. ¨ Repeat with a new element.

Ch. 4 - Atomic Structure III. Masses of Atoms ¨ Atomic Mass ¨ Mass Number ¨ Isotopes

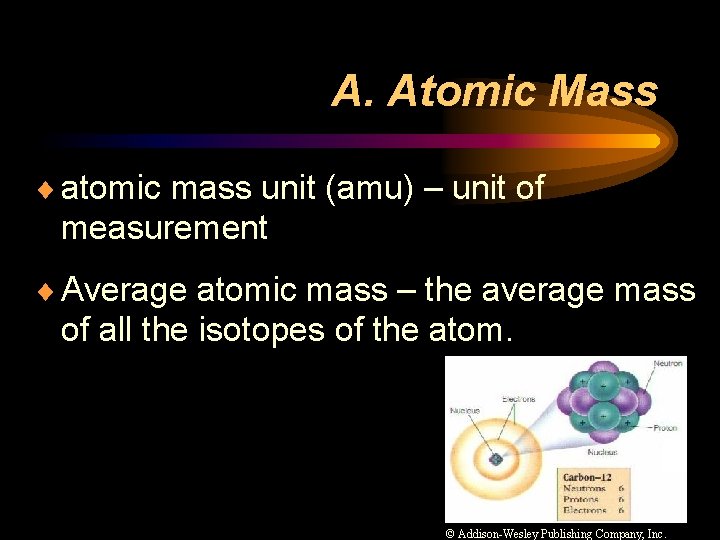
A. Atomic Mass ¨ atomic mass unit (amu) – unit of measurement ¨ Average atomic mass – the average mass of all the isotopes of the atom. © Addison-Wesley Publishing Company, Inc.

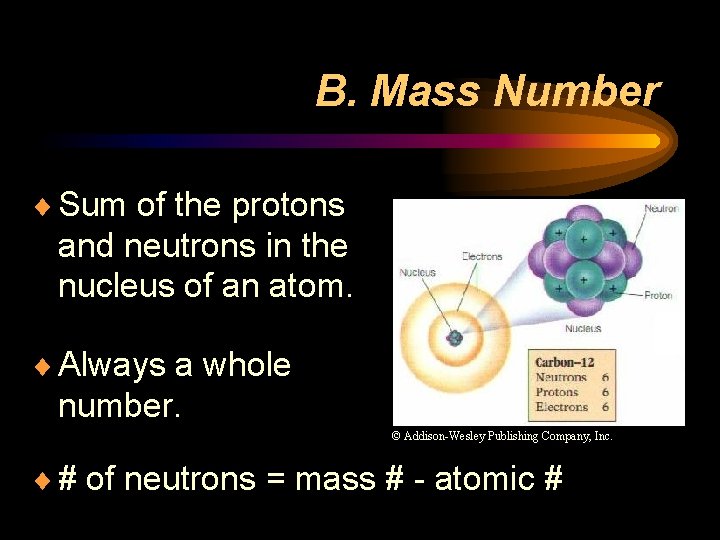
B. Mass Number ¨ Sum of the protons and neutrons in the nucleus of an atom. ¨ Always a whole number. © Addison-Wesley Publishing Company, Inc. ¨ # of neutrons = mass # - atomic #

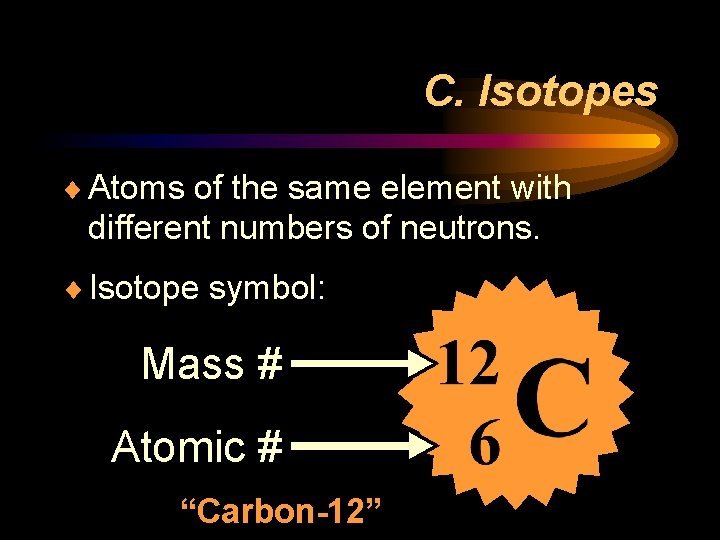
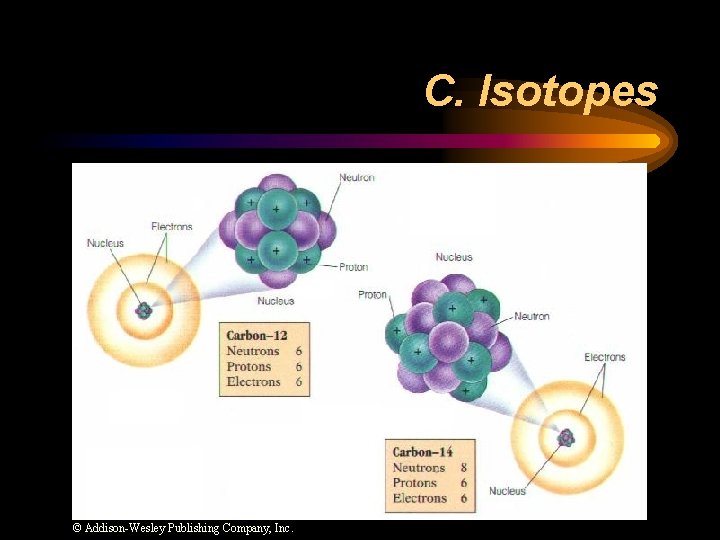
C. Isotopes ¨ Atoms of the same element with different numbers of neutrons. ¨ Isotope symbol: Mass # Atomic # “Carbon-12”

C. Isotopes © Addison-Wesley Publishing Company, Inc.